Rebescensine A powder injection and preparing process thereof
A technology for oridonin A and freeze-dried powder injection, which is applied in the field of medicine, can solve the problems of complex preparation process, inability to realize industrialized production and the like, and achieve the effects of enhancing in vivo stability, simple composition and high efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
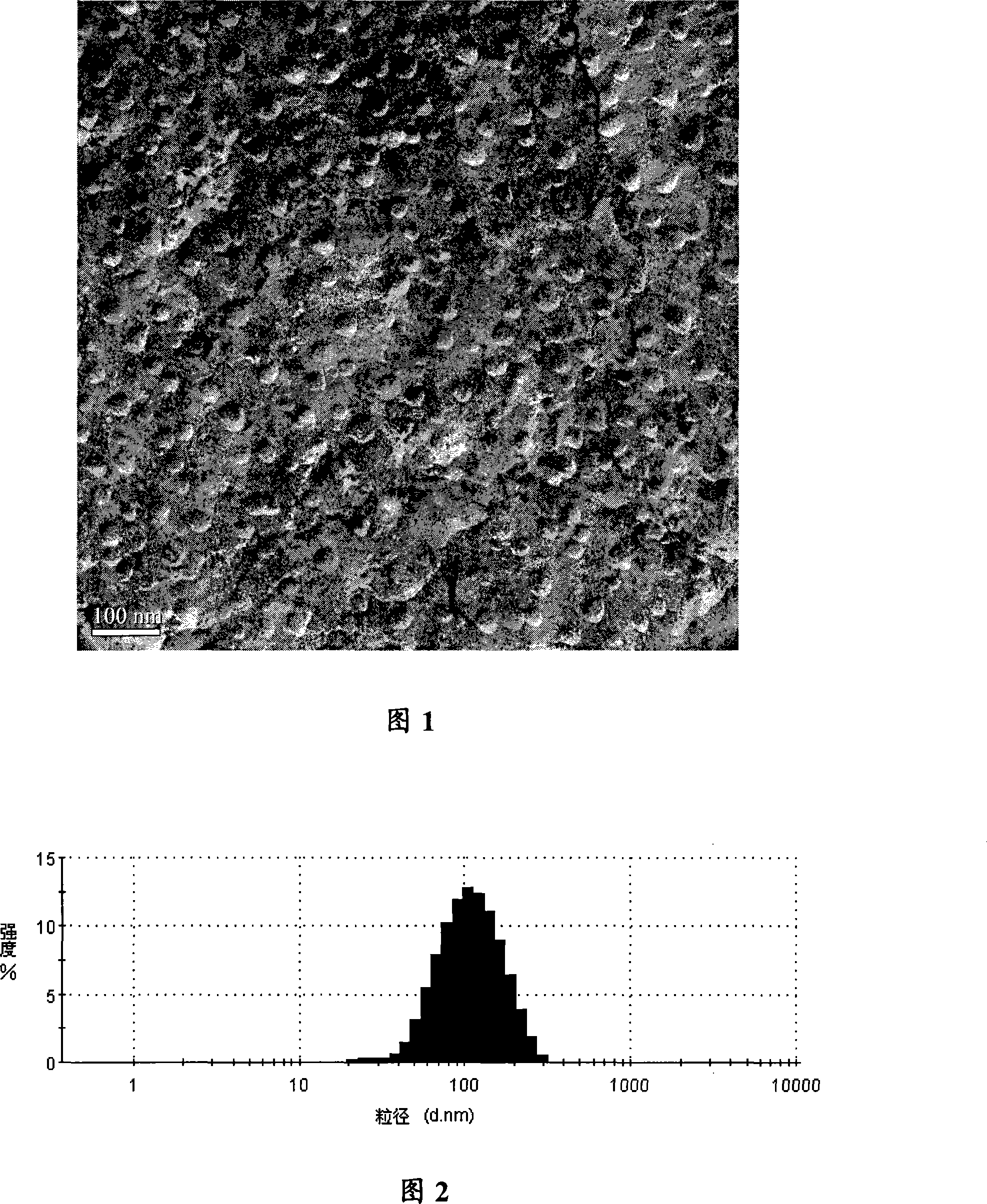
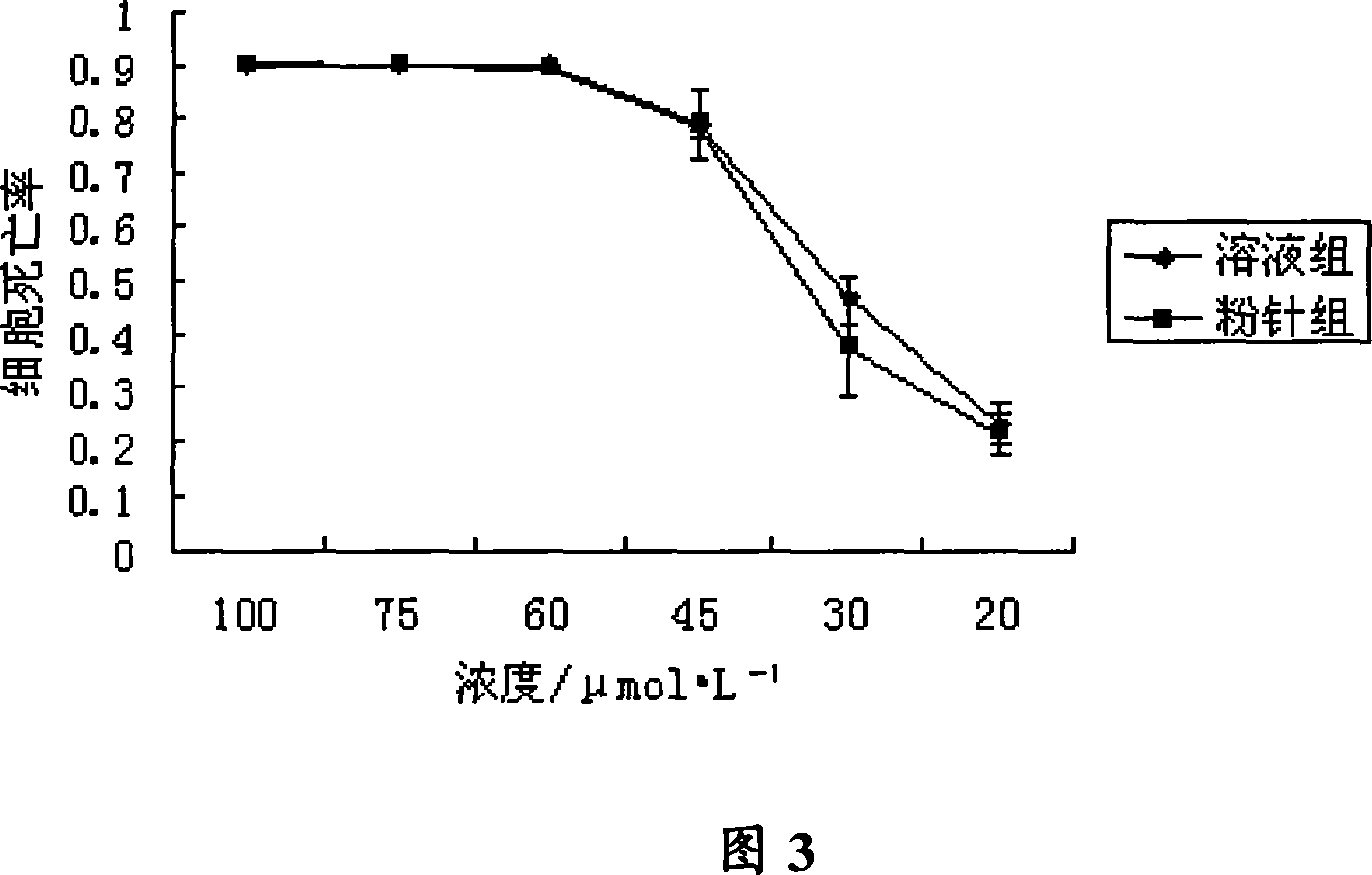
Image
Examples
Embodiment 1
[0029] Dissolve 1200g of glucose, 300g of dextran in about 10L of distilled water, 60g of oridonin, and 100g of soybean lecithin in an appropriate amount of ethanol, and drop them into the above water phase under stirring. Heat and continue to stir or evaporate part or all of the organic solvent under reduced pressure, and set the volume to 10L. Then use a 0.22μm microporous membrane to filter and sterilize, divide into 16.6ml each, and freeze-dry at -10~-55°C. During the freeze-drying process, remove the residual solvent, cover the rubber stopper, and roll the cap to get each tube. 100mg (oridonin active ingredient) freeze-dried powder injection.
Embodiment 2
[0031] 50g of lecithin, dissolved in about 10L of distilled water, 50g of oridonin, dissolved in an appropriate amount of ethanol, and injected into the aqueous phase obtained above under stirring conditions, heated and stirred or evaporated part or all of the organic solvent under reduced pressure, Add 600g of glucose, dissolve 200g of dextran, and dilute to 10L. Use a 0.22μm microporous membrane to filter and sterilize, divide into 20ml each, freeze-dry at -10~-55°C, remove residual solvent during the freeze-drying process, cover with rubber stopper, and crimp the cap to obtain 100mg per vial ( oridonin active ingredient) freeze-dried powder injection.
Embodiment 3
[0033] Oridonin 80g, Poloxamer 188 or Poloxamer F68 160g was dissolved in an appropriate amount of ethanol, and slowly poured into about 20L of distilled water while stirring. Heat and continue to stir or evaporate part or all of the organic solvent under reduced pressure, add 2000 g of dextran, and set the volume to 20 L. Then use a 0.22μm microporous membrane to filter and sterilize, pack into 25ml each, freeze-dry at -10~-55°C, remove residual solvent during the freeze-drying process, cover with rubber stopper, and roll the cap to obtain 100mg per tube (Oridonin A active ingredient) freeze-dried powder injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com