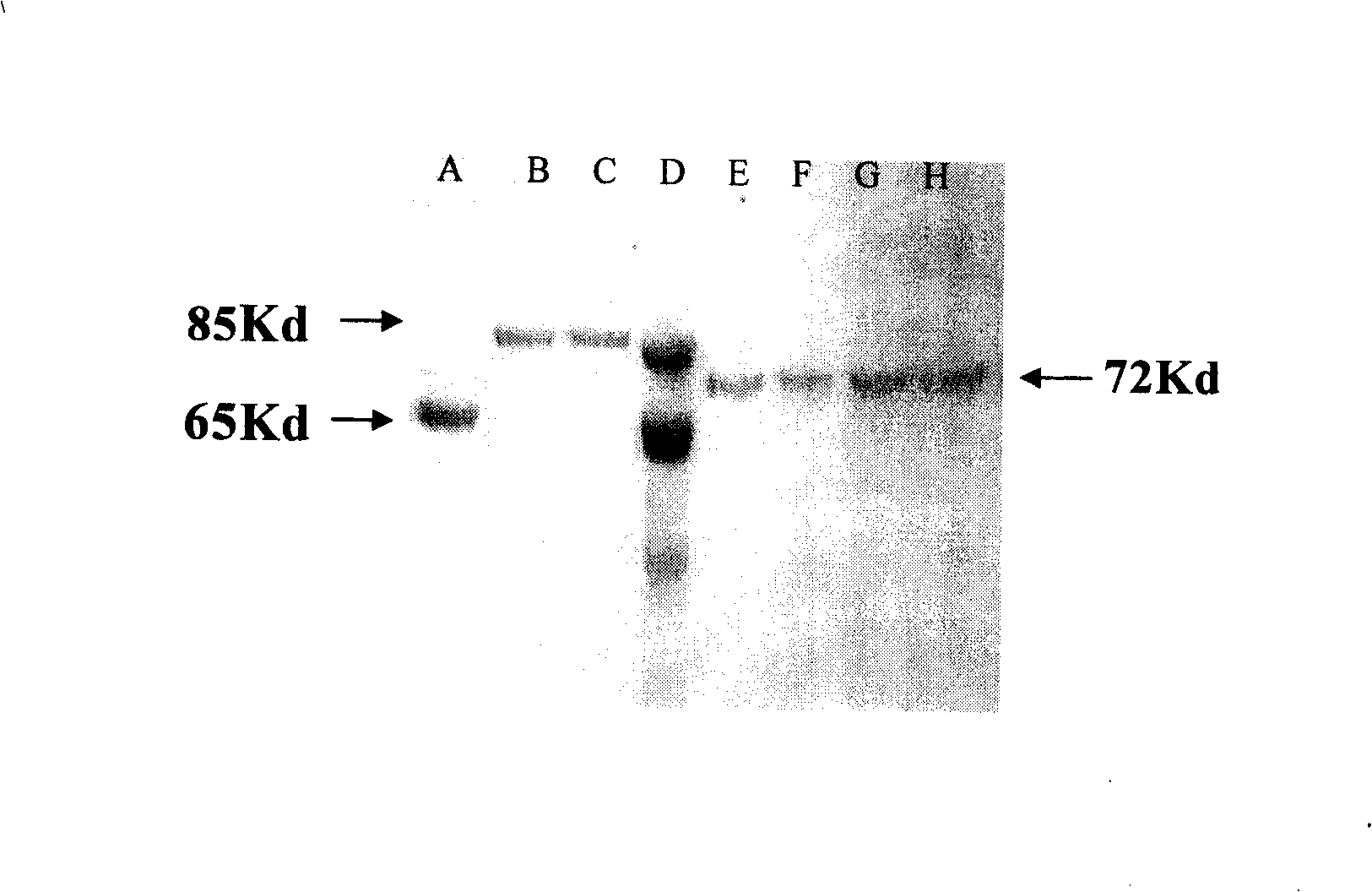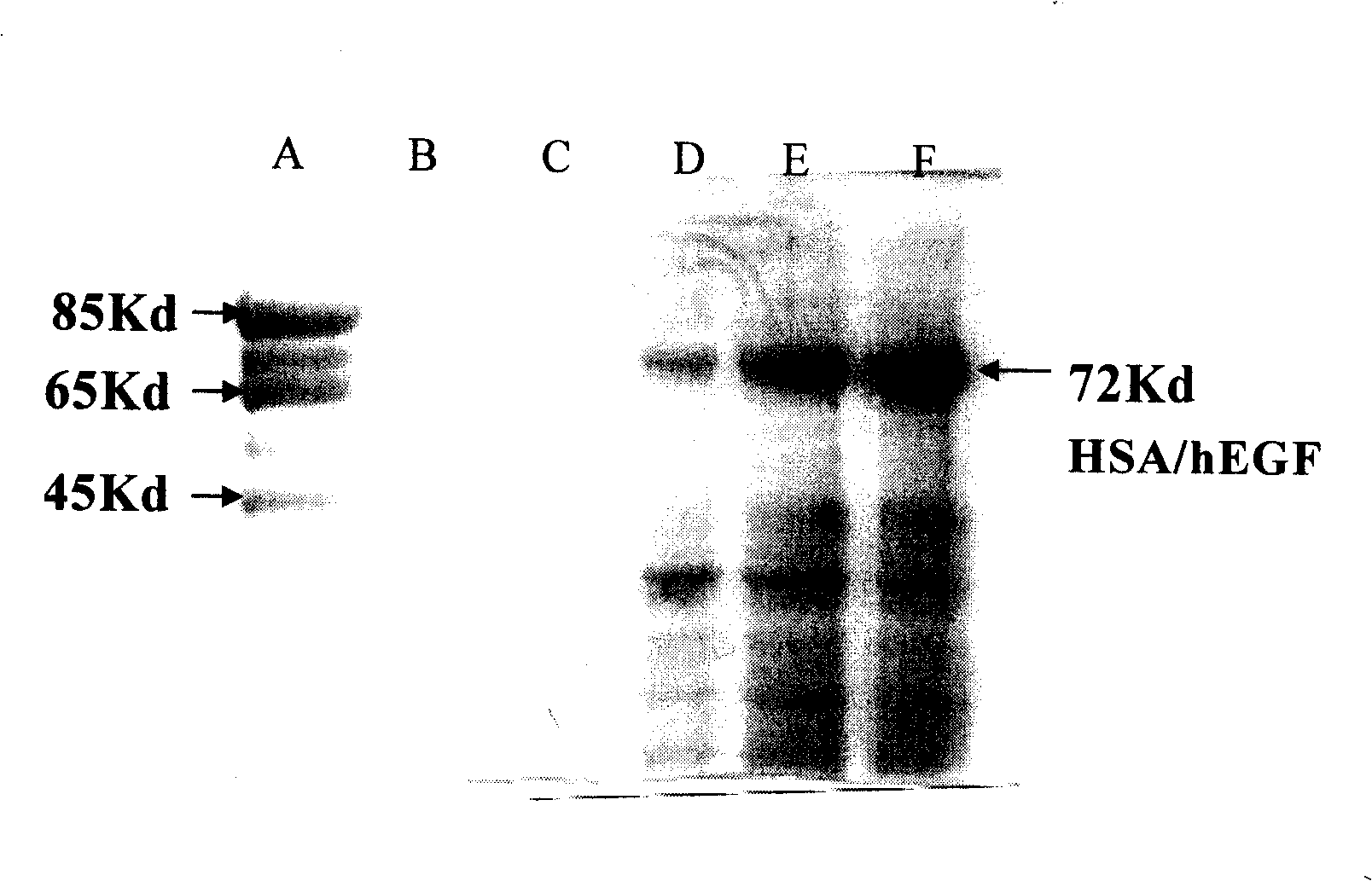Technique for preparing amalgamation protein skin-protection product containing albuminar and skin cell growth factor, and uses of the same
A technology of human serum albumin and fusion protein, applied in the field of preparation and application of fusion protein skin care products containing human serum albumin and skin cell growth factor, which can solve the problems of short lifespan in vitro and meet the requirements of storage conditions and transportation The effect of reducing, reducing cost, reducing dosage and frequency of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the fermentation process of HSA fusion protein recombinant yeast
[0049] Several genes containing the human serum albumin gene to be expressed, the fusion gene of human serum albumin and KGF, the fusion gene of human serum albumin and bFGF, or the fusion gene of human serum albumin and hEGF (CGMCC No. 2072) or human serum albumin and hIGF fusion gene yeast colonies were cultured in basic culture medium containing Zeocin antibiotics, buffer capacity and glycerin. Cultivate on a temperature-controlled shaker at a speed of 300 rpm until the cell density reaches OD 600 =2-6. The culture was centrifuged at 1500 rpm for 15 minutes to collect the bacteria, and the bacteria were resuspended in the same basic culture medium but without glycerol, and changed to 0.5% methanol, and the culture continued until the cell density reached OD 600 = 1.0. Under the induction of methanol, the yeast starts to express the foreign protein under the action of the promoter. Th...
Embodiment 2
[0052] Embodiment 2: Separation and purification technology of HSA fusion protein
[0053] The culture supernatant expressed by recombinant yeast cells (ZY-HSA / GF) or CHO mammalian cells contains the fusion protein HSA / GF of serum albumin secreted. After centrifuging and separating the bacteria, the supernatant was treated with 0.4-1% activated carbon, collected, concentrated, and the salt concentration was reduced, then the pH was adjusted to about 7.5, and the concentrated solution was passed through the Affi-Gel Blue-Gel chromatography column manufactured by Bio-Rad Company . HSA or HSA / GF is combined with the functional group on the chromatography column and hung on the column. After washing, HSA or HSA / GF can be eluted through a 1-5M NaCl gradient to obtain a protein preparation with a purity of 75-85%. If necessary, it can be further passed through a molecular sieve chromatography column to obtain a protein preparation with a purity increased to 95-99% or more. For th...
Embodiment 3
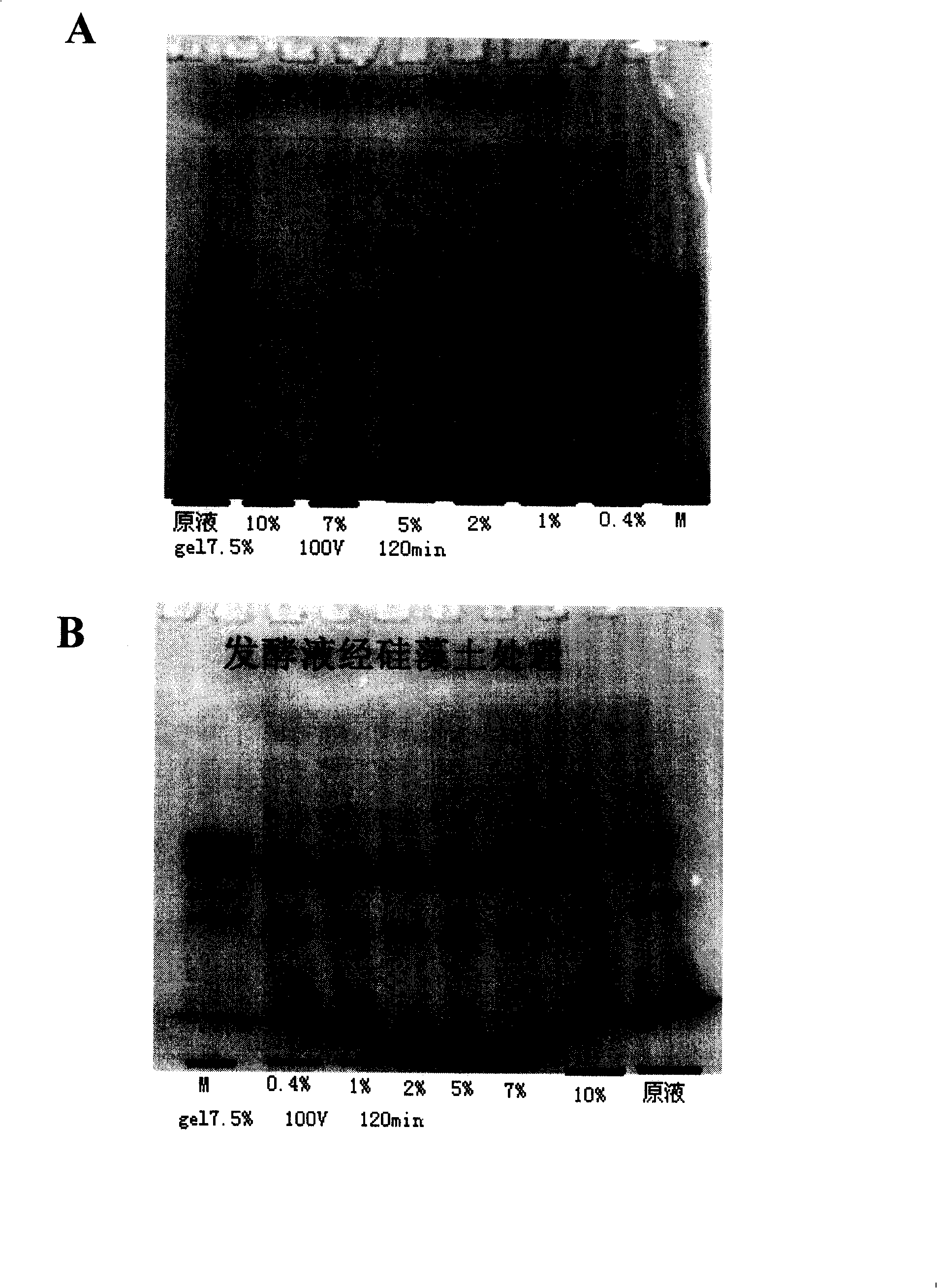
[0054] Embodiment 3: the preparation technology of the effective component of the fermentation liquid of HSA fusion protein
[0055] If the treated fermentation supernatant is directly used for the preparation of cosmetics, it should be treated with 0.1%-10% activated carbon or diatomaceous earth. The dosage of activated carbon or diatomaceous earth is determined according to the yeast pigment (A350 / 280 absorbance value) in the fermentation broth. Preferably, 0.4%, more preferably 2% activated carbon. After the fermentation broth is decolorized, use a 20K concentration bag to concentrate to 10% of the original volume of the fermentation broth. Then desalting and changing the liquid into PB buffer solution on G25 according to the column volume ratio of 30-50%, thus obtaining the functional component (GX component) which can be used as cosmetic preparation. The GX component contains a large amount of fusion protein, yeast protein, fusion protein fragments, zymosan, etc., which...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com