Method of producing high-purity anhydrous lithium chloride
A lithium chloride, high-purity technology, applied in the direction of alkali metal chloride, etc., can solve the problems of difficult separation of impurities potassium and sodium, high production cost, unreasonable economy, etc., and achieve the effect of low cost, easy operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
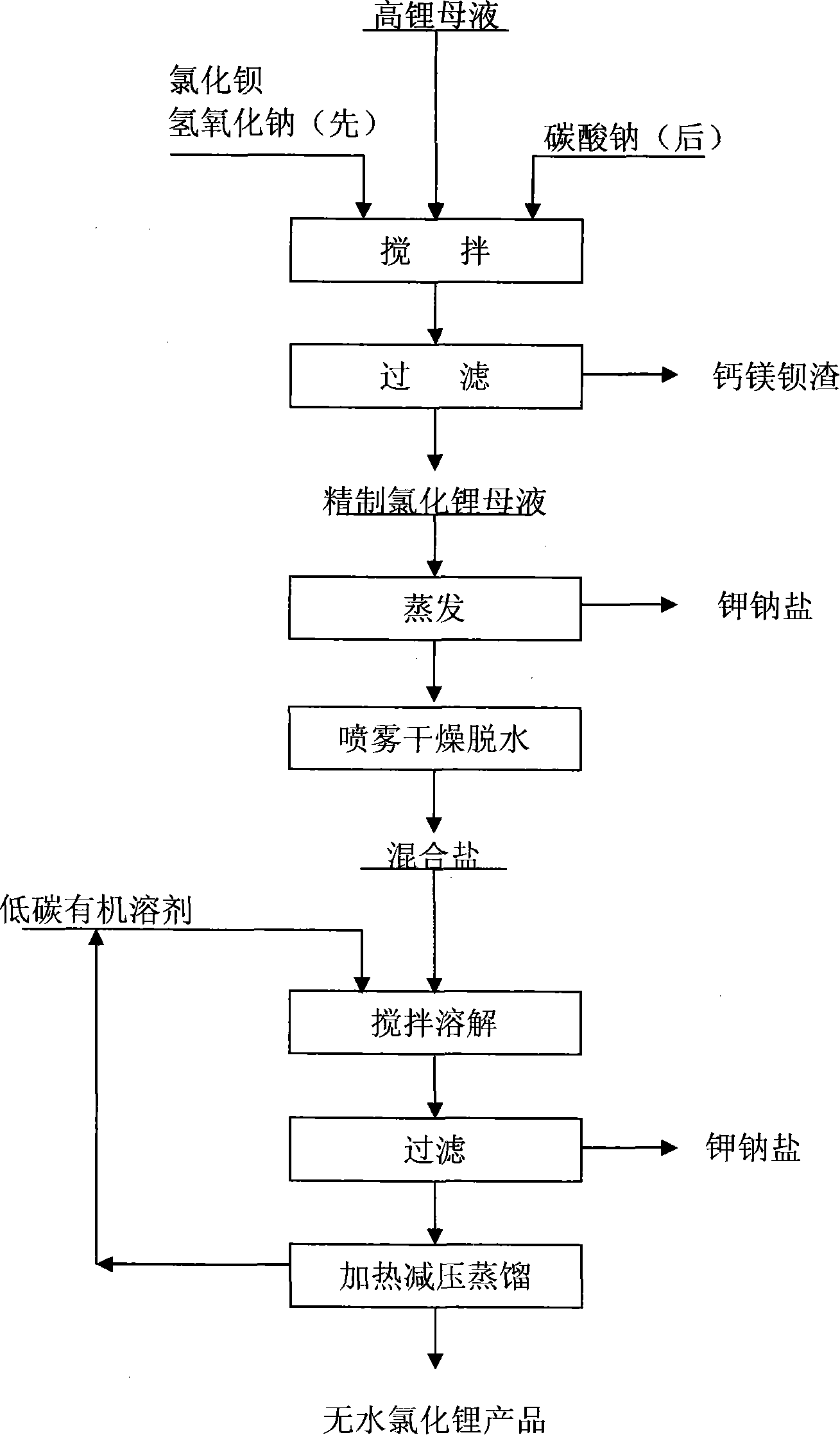
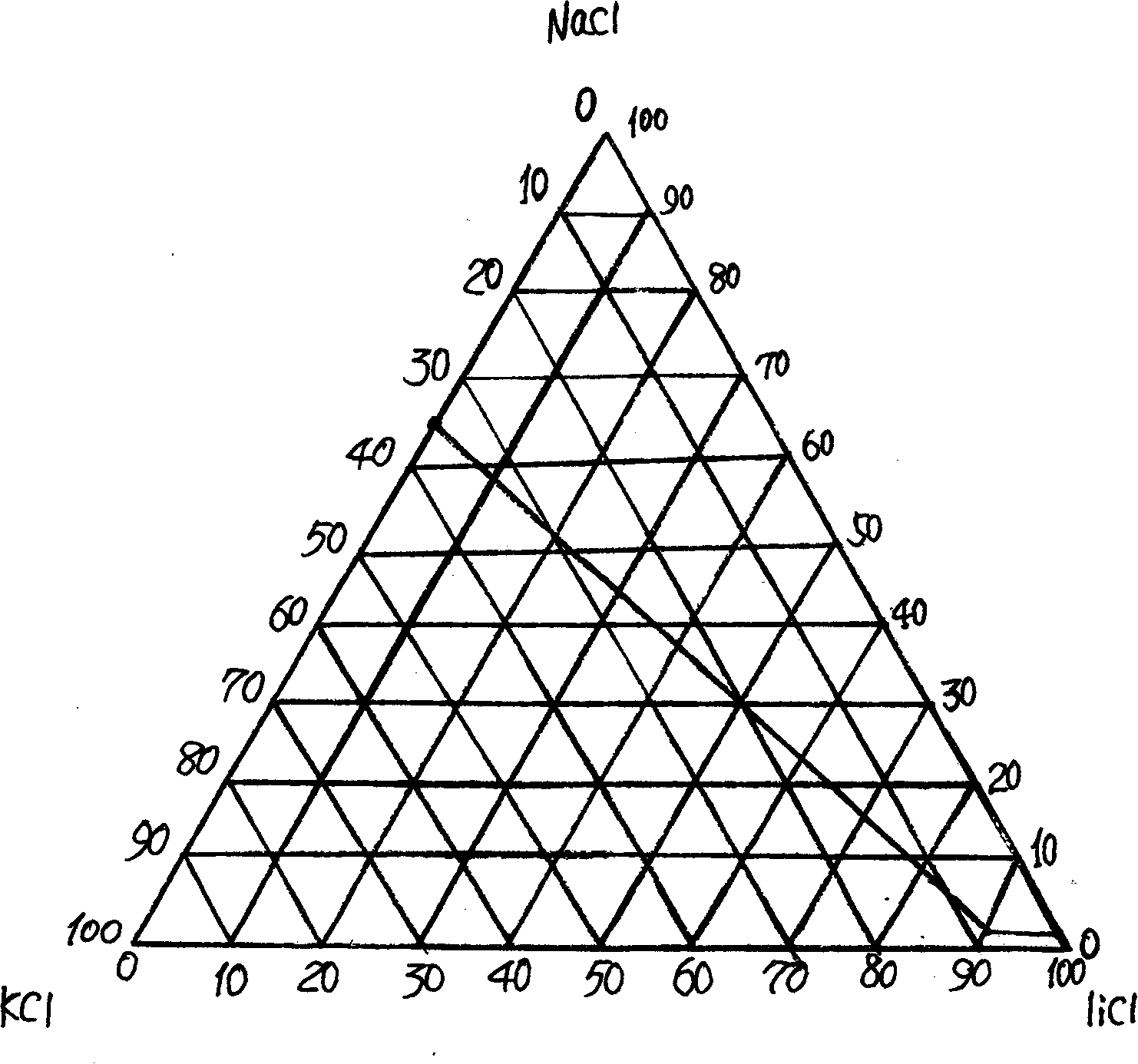
[0016] Add sodium hydroxide and barium chloride to low-magnesium, high-potassium sodium brine containing lithium chloride, and stir to precipitate magnesium ions and sulfate ions to obtain a mother liquor containing lithium chloride; then add a certain amount of sodium carbonate solution, Filtration and separation to remove traces of Ca 2+ , Ba 2+ ions to obtain a refined lithium chloride mother liquor, evaporate the mother liquor to close to the co-saturation point to separate a large amount of sodium chloride and potassium chloride, and spray dry the mother liquor to obtain a high-lithium mixed salt containing NaCl, KCl, and LiCl, wherein the LiCl content At 60-95%, weigh 100g of dry lithium mixed salt, including 93.18% of lithium chloride, 2.87% of sodium chloride, and 3.92% of potassium chloride, add it to 150ml of absolute ethanol, and stir at room temperature for 60 minutes under airtight conditions Filter to obtain the filtrate of lithium chloride; move the filtrate to...
Embodiment 2
[0018] Add sodium hydroxide and barium chloride to low-magnesium, high-potassium sodium brine containing lithium chloride, and stir to precipitate magnesium ions and sulfate ions to obtain a mother liquor containing lithium chloride; then add a certain amount of sodium carbonate solution, Filtration and separation to remove traces of Ca 2+ , Ba 2+ ions to obtain a refined lithium chloride mother liquor, evaporate the mother liquor to close to the co-saturation point to separate a large amount of sodium chloride and potassium chloride, and spray dry the mother liquor to obtain a high-lithium mixed salt containing NaCl, KCl, and LiCl, wherein the LiCl content At 60-95%, weigh 100g of dry lithium mixed salt, including lithium chloride 93.18%, sodium chloride 2.87%, potassium chloride 3.92%, add 300ml absolute ethanol and 50ml acetone mixed solution, under airtight condition, Stir at room temperature for 30 minutes and filter to obtain the filtrate of lithium chloride; transfer t...
Embodiment 3
[0020] Add sodium hydroxide and barium chloride to low-magnesium, high-potassium sodium brine containing lithium chloride, and stir to precipitate magnesium ions and sulfate ions to obtain a mother liquor containing lithium chloride; then add a certain amount of sodium carbonate solution, Filtration and separation to remove traces of Ca 2+ , Ba 2+ ions to obtain a refined lithium chloride mother liquor, evaporate the mother liquor to close to the co-saturation point to separate a large amount of sodium chloride and potassium chloride, and spray dry the mother liquor to obtain a high-lithium mixed salt containing NaCl, KCl, and LiCl, wherein the LiCl content At 60-95%, weigh 100g of dry lithium mixed salt, including lithium chloride 93.18%, sodium chloride 2.87%, potassium chloride 3.92%, add 850ml absolute ethanol and 150ml methanol mixed solution, under airtight condition, Stir at room temperature for 10 minutes and filter to obtain the filtrate of lithium chloride; transfer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com