Dual quantification method and reagent kit for stable isotope 18 O marked proteome
A stable isotope and proteome technology, applied in measuring devices, biological testing, material inspection products, etc., can solve the problem of high price of Lys-N enzyme, and achieve the effect of wide practicability, easy realization and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
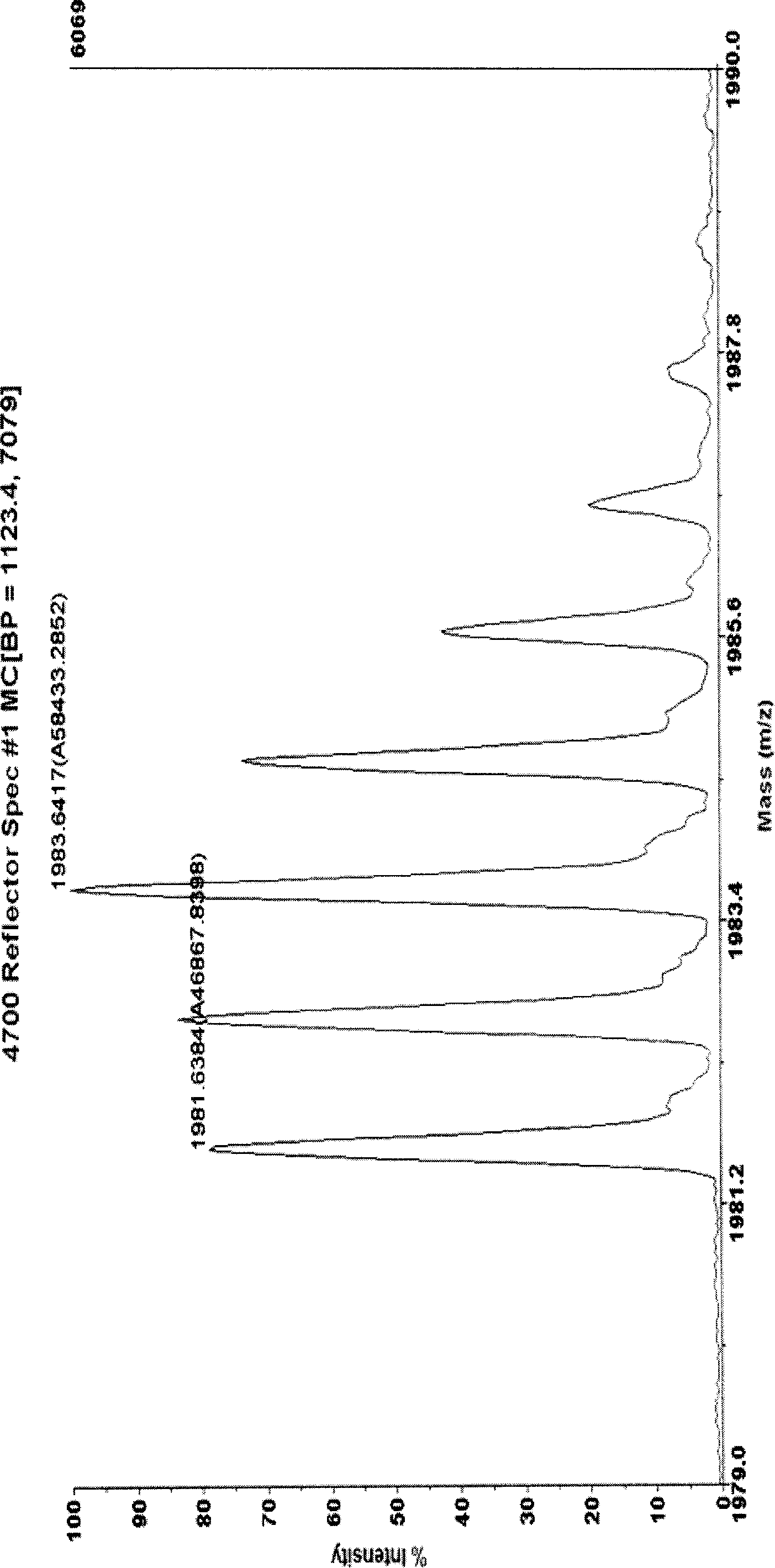
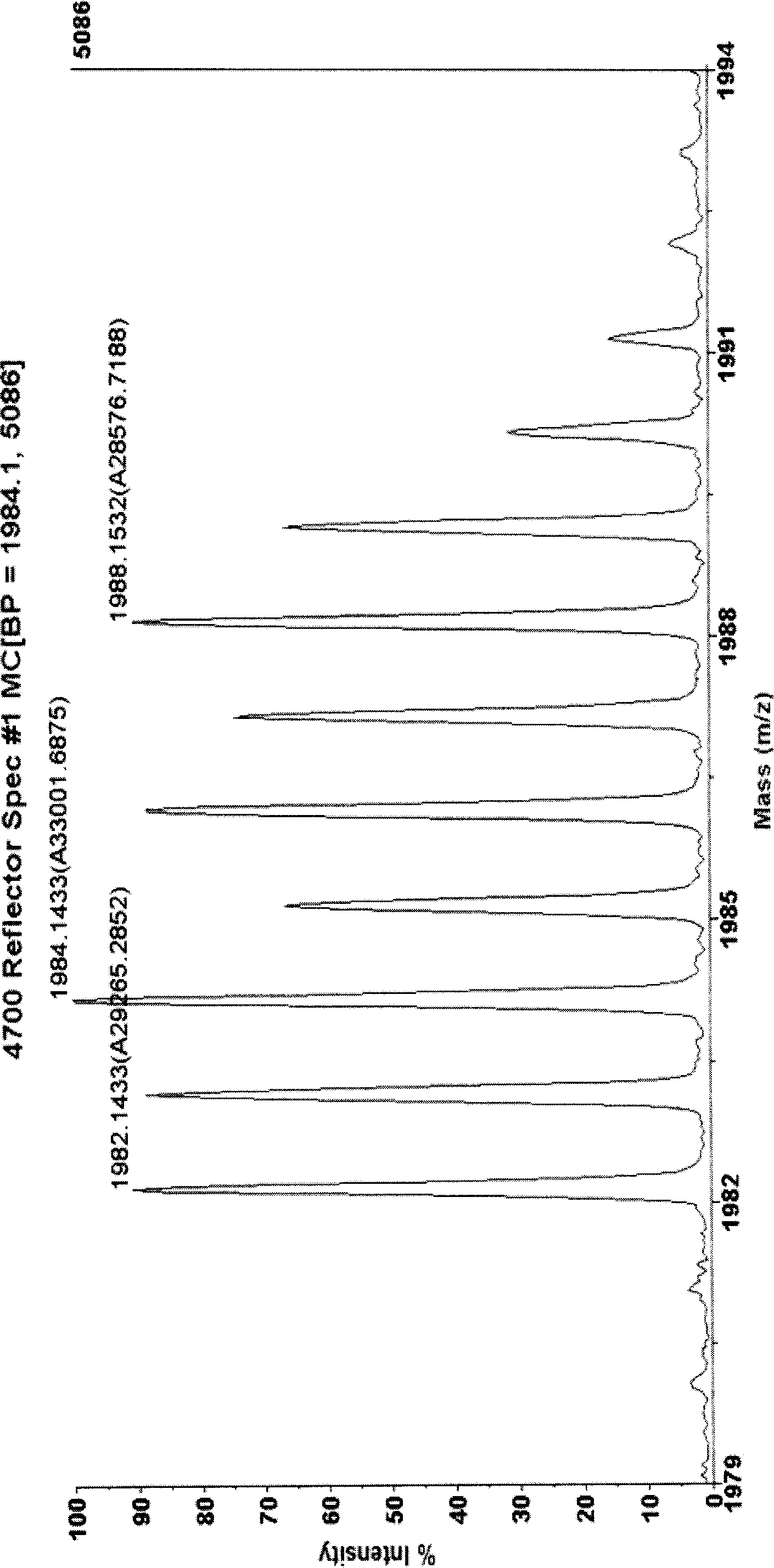
[0036] Example 1 Double quantitative labeling of horse myoglobin
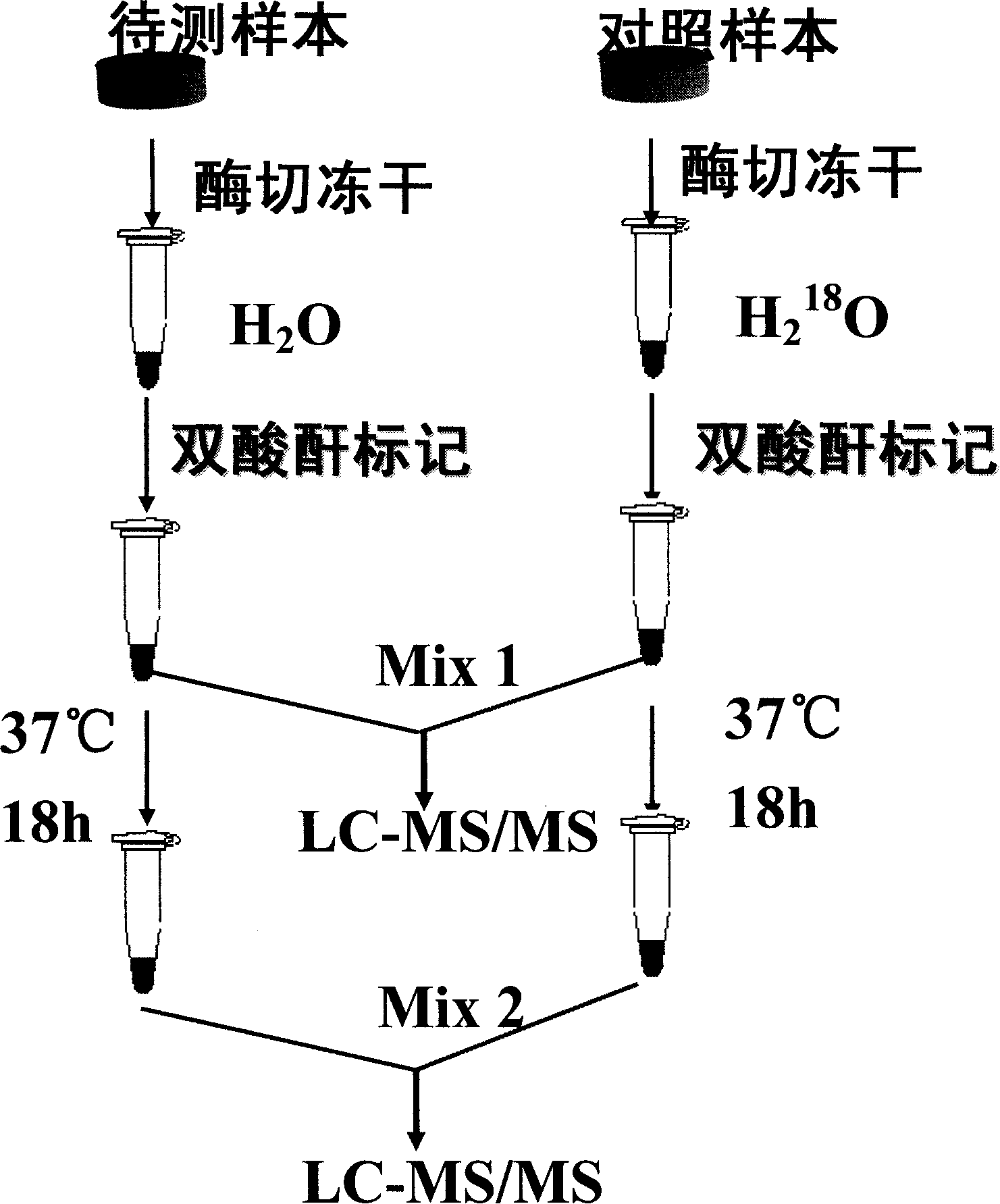
[0037] 1.1 Take 0.1 mg of horse myoglobin and dissolve it in 100 μL of 50 mM pH7.0 triethylamine bicarbonate buffer solution. Denature the protein by heating at 95°C for 5 minutes. Myoglobin has no cysteine residues in its amino acid sequence, so a reductive alkylation step is not required. Add 2ug trypsin, digest at 37°C for 2 hours. Then add 2ug of trypsin, and digest at 37°C for 10 hours. Divide the myoglobin peptides obtained by enzymatic digestion into four parts, two of which were heated in a boiling water bath for ten minutes and then cooled rapidly in an ice bath to inactivate trypsin. Then dry completely in a vacuum dryer with the remaining two copies. In the obtained solid powder, take one part of trypsin-inactivated and one part of trypsin-inactivated sample and dissolve it in 25 μL 18 50mM triethylamine bicarbonate buffer solution with pH 7.0 in O water. The remaining two aliquots were disso...
Embodiment 2 6
[0042] The double quantification of embodiment 2 six kinds of standard protein mixtures
[0043] 2.1 Six standard protein mixtures were purchased from Sigma, USA, and configured according to the following proportions (mixtures A and B, μg / mL): β-casein (10, 10); β-lactoglobulin (10, 40) ; myoglobin (60, 20); bovine serum albumin (40, 20); lysozyme (50, 50); transferrin (30, 60). The protein mixture was dissolved in 50 μL pH7.0, 50 mM triethylamine bicarbonate solution, and denatured by heating at 95 ° C for 5 min. Add 2 μL of 50 mM reducing agent tricarboxyethyl phosphine, and incubate at 60° C. for 1 h. Then 2 μL of 250 mM alkylating reagent iodoacetamide was added, left in the dark for 10 minutes, and trypsin was added according to the ratio (1:20w / w), 37°C, 18h. Vacuum-dry the protein peptide solution obtained by enzymatic digestion, add 18 The triethylamine bicarbonate buffer solution prepared with O water was dissolved, and reacted with 5-fold excess bis-acid anhydride...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com