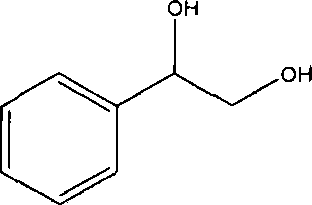
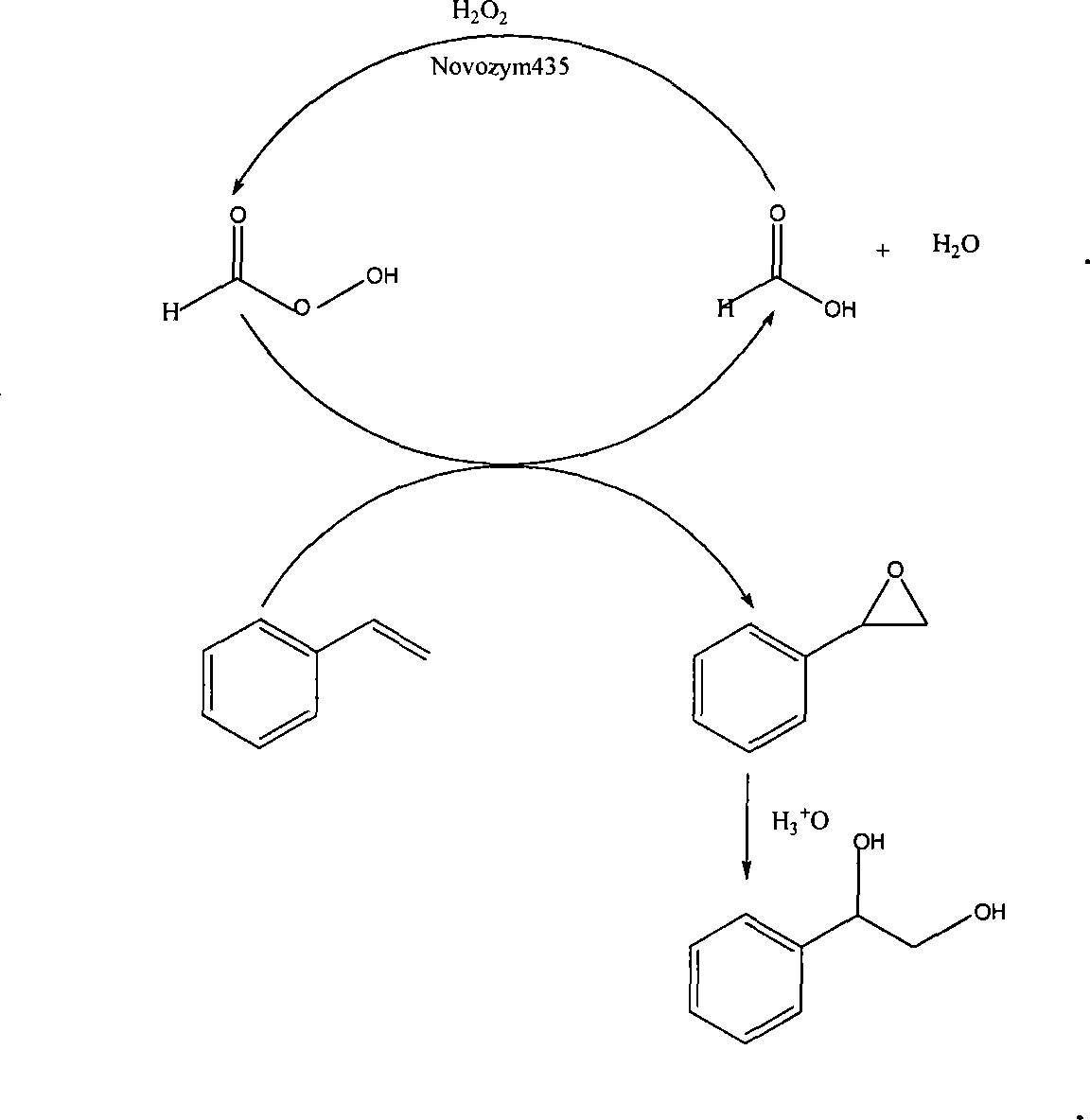
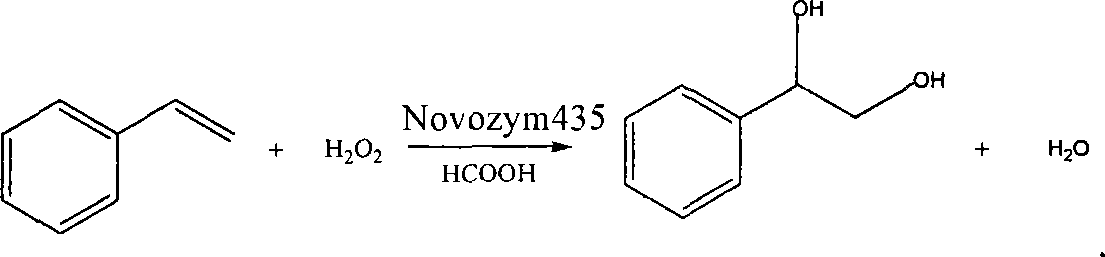Method of lipase-catalyzed preparing 1-phenyl-1, 2-ethanediol
A technology for catalytic preparation of ethylene glycol, which is applied in fermentation and other fields, can solve the problems of severe equipment corrosion, complicated process steps, and bromide application restrictions, and achieve the effects of saving raw materials, mild reaction conditions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In a 100ml flask, add 5ml (0.043mol) styrene, 1ml formic acid, 3ml solvent benzene, 0.0500g catalyst Novozym435;
[0023] Slowly start adding 5ml of 15% hydrogen peroxide dropwise in a constant temperature water bath with a magnetic stirring temperature of 30°C, complete the dropwise addition in 1 hour, and then continue the reaction at 30°C for 18 hours;
[0024] After the reaction was completed, 10ml of ethyl acetate was added to extract the layers, and the organic layer was washed with anhydrous Na 2 SO 4 dry;
[0025] Rotary steaming under reduced pressure at 60°C can obtain 1-phenyl-1,2-ethanediol with a content of 75.69%, and the conversion rate of styrene is 94.3%.
Embodiment 2
[0027] In a 100ml flask, add 3ml solvent benzene, 5ml (0.043mol) styrene, 2ml formic acid, 0.0500g catalyst Novozym435;
[0028] Slowly start adding 7.5ml of 30% hydrogen peroxide dropwise in a constant temperature water bath with a magnetic stirring temperature of 50°C, and the dropwise addition is completed in about 1 hour, and then continue to react at a temperature of 50°C for 23 hours;
[0029] Add 10ml of ethyl acetate after the reaction to extract and separate the layers, and the organic phase is washed with anhydrous Na 2 SO 4 dry;
[0030] Finally, rotary evaporation under reduced pressure at 60°C can obtain 1-phenyl-1,2-ethanediol with a content of 70.67%, and the conversion rate of styrene is 96.4%.
Embodiment 3
[0031] Embodiment 3 (solvent-free operation)
[0032] Add 5ml (0.043mol) styrene, 0.5ml formic acid, 0.0500g catalyst Novozym435 in 100ml flask;
[0033] Slowly start adding 5ml of 40% hydrogen peroxide dropwise in a constant temperature water bath with a magnetic stirring temperature of 45°C, and the dropwise addition is completed in about 1 hour, and then continue to react at a temperature of 45°C for 30 hours;
[0034] Add 10ml of ethyl acetate after the reaction to extract and separate the layers, and the organic phase is washed with anhydrous Na 2 SO 4 dry;
[0035] Finally, rotary evaporation under reduced pressure at 60°C can obtain 1-phenyl-1,2-ethanediol with a content of 56.68%, and the conversion rate of styrene is 91.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com