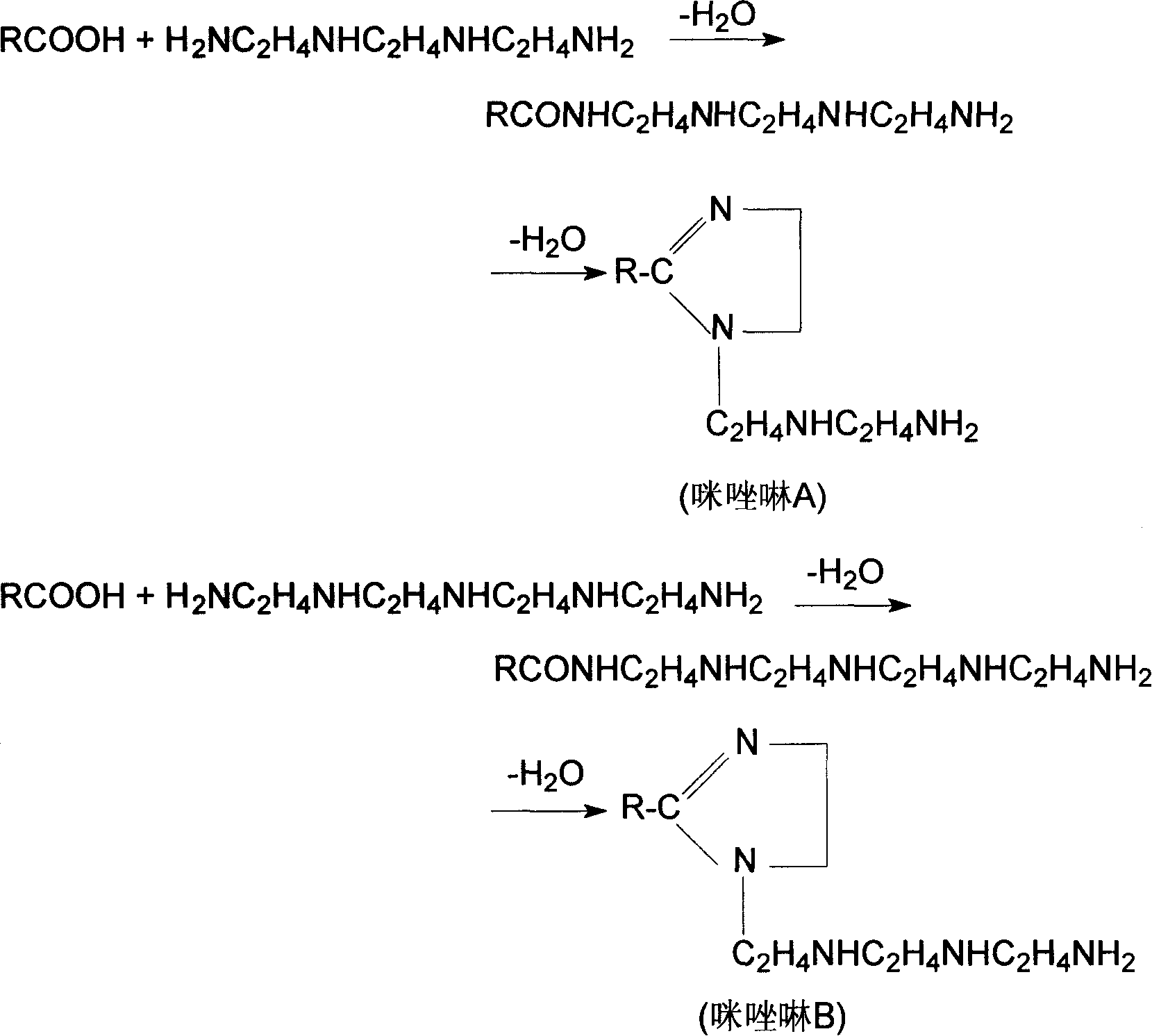
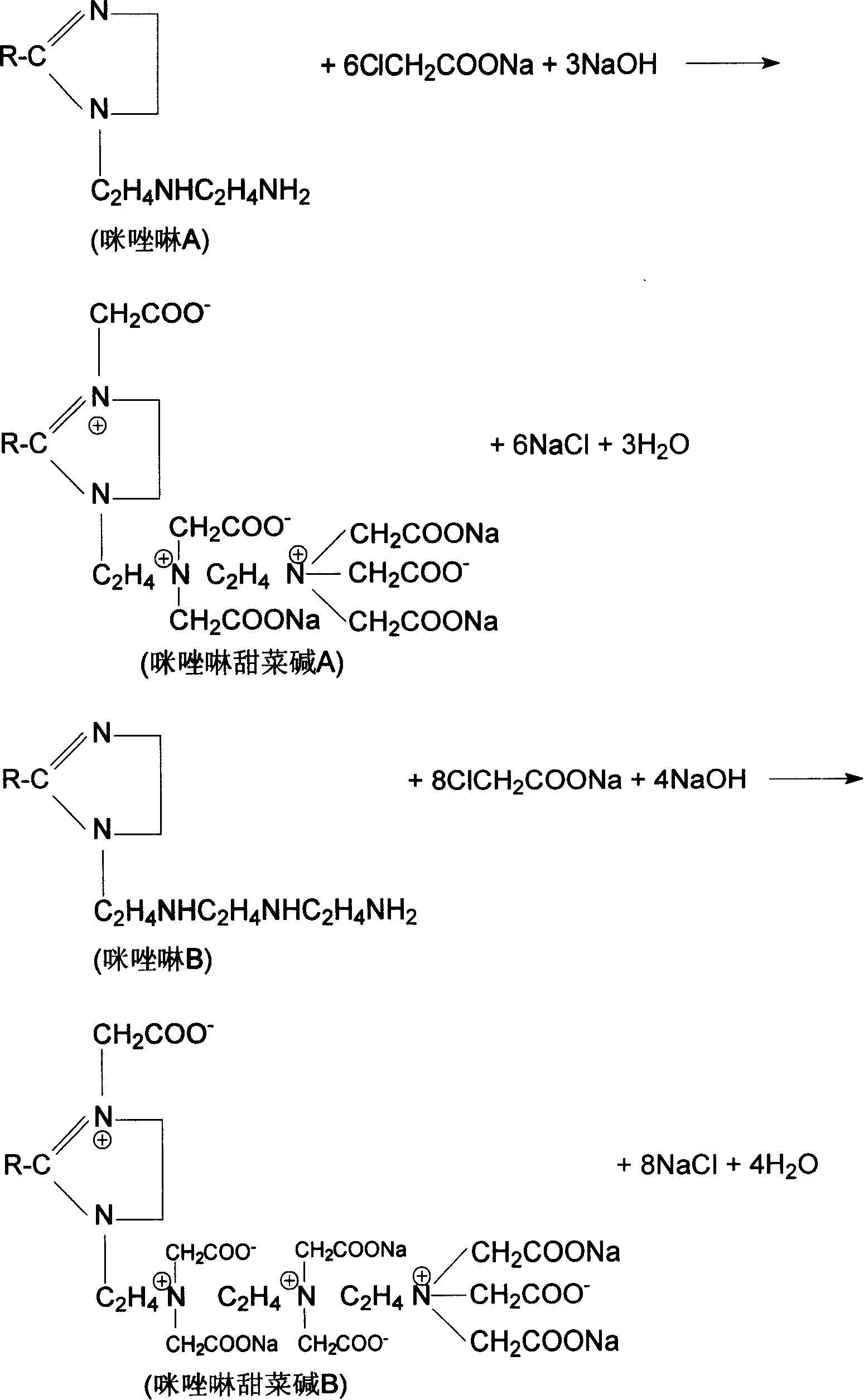
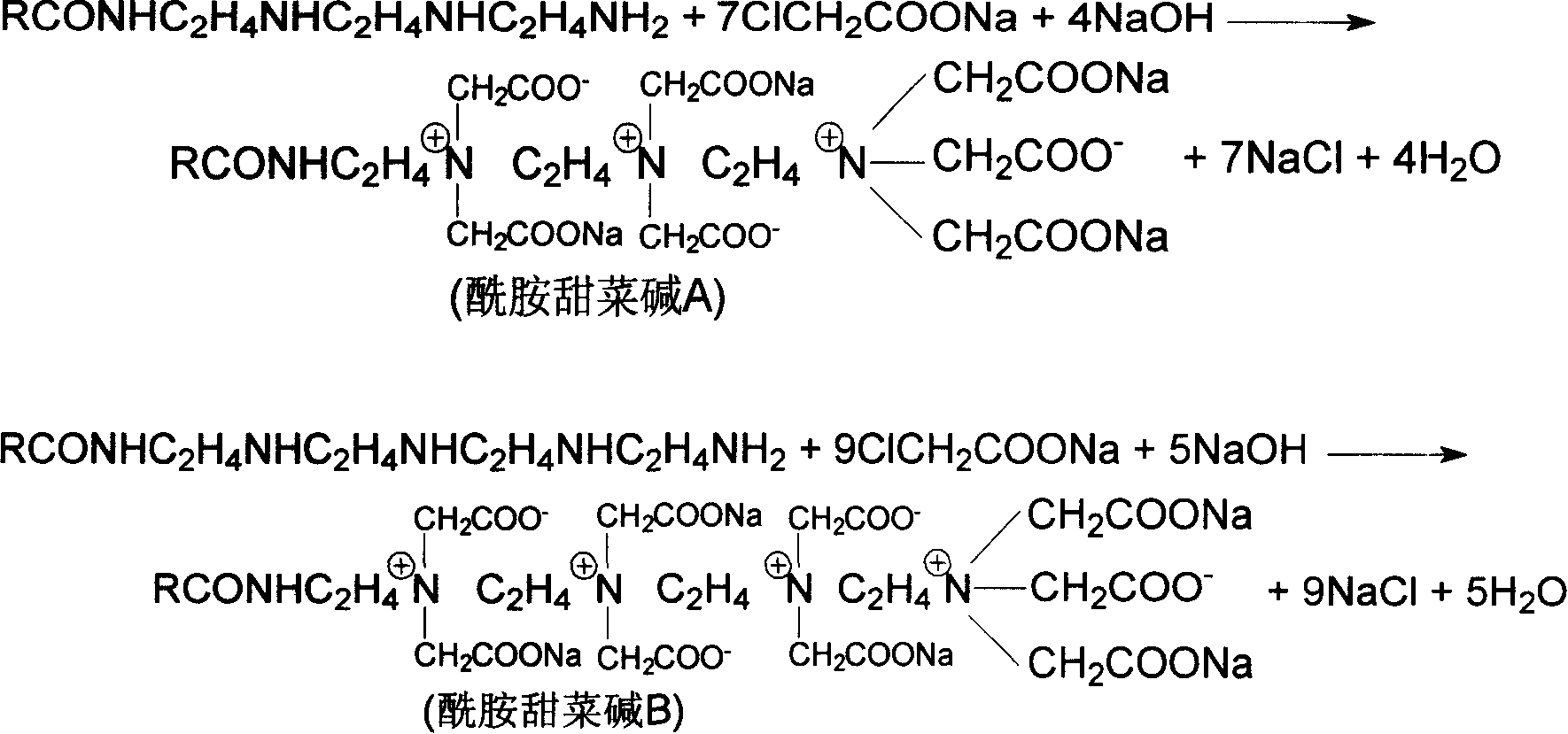Method for preparing naphthenic acid imidazolines and betaine of amide thereof, and application of the same in HCl-H2S-H2O system
A technology of naphthenic acid imidazoline and betaine, applied in the direction of organic chemistry, etc., can solve the problems of waste of ammonia water, large pH fluctuation range, inability to effectively inhibit corrosion, etc., and achieves simplified process operation, reduced product cost, The effect of excellent corrosion inhibition performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] In a 500mL four-necked flask equipped with a stirring, dropping funnel, thermometer and reflux condenser, add the newly synthesized and cooled to 109°C made of naphthene with an acid value of 5.60 mgKOH / g and an amine value of 267.30 mgKOH / g Naphthenic acid imidazoline (abbreviated as imidazoline A, the same below) 75.5 g (equivalent to 0.120 moles of cycloalkyl) synthesized by acid and triethylenetetramine, according to the cycloalkyl contained in the naphthenic imidazoline and chloroacetic acid solution The molar ratio of the contained chloroacetic acid is 1.0:4.1, and 103.3 g of a prepared 45.0% chloroacetic acid solution (0.492 moles of chloroacetic acid) is added under stirring, and then the temperature is gradually raised under stirring. At the beginning, the reaction speed is faster, and the reaction temperature is controlled at a lower 60-70°C. As the reaction proceeds, the pH value of the reaction solution decreases. To ensure the continuation of the reaction, ...
Embodiment 2
[0053] In the same four-necked flask as in Example 1, add 38.20% sodium chloroacetate solution 183.0 g (sodium chloroacetate 0.60 moles), press the cycloalkyl group contained in the naphthenic acid imidazoline and the chloroacetic acid contained in the sodium chloroacetate solution The molar ratio of sodium is 1.0:10.0, and 37.8 g of imidazoline A (equivalent to 0.06 moles of cycloalkyl) is added under stirring, and then the temperature is gradually raised under stirring, and the reaction temperature is controlled at 50-60°C. As the reaction proceeds, the pH value of the reaction solution decreases. To ensure the continuation of the reaction, the pH is controlled to be 7-8.5 by dropwise adding 30% NaOH solution. Simultaneously, as the reaction proceeds, the reaction rate will decrease. In order to ensure the drop rate of NaOH solution at a pH value of 7 to 8.5, the reaction temperature will gradually increase. Finally, rise to 85-90°C. When the reaction reached 9h, the dropwi...
Embodiment 3
[0056] In the same four-necked flask as in Example 1, add 38.20% sodium chloroacetate solution 155.5g (0.51 moles of sodium chloroacetate), press the cycloalkyl group contained in the naphthenic acid imidazoline and the chloroacetic acid contained in the sodium chloroacetate solution The molar ratio of sodium is 1.0:5.1, and under stirring, add naphthenic acid imidazoline (referred to as imidazoline) synthesized by naphthenic acid and tetraethylenepentamine with an acid value of 4.61 mgKOH / g and an amine value of 297.32 mgKOH / g. B, the same below) 75.5g (equivalent to cycloalkyl 0.10 mole), then gradually heat up under stirring. At the beginning, the reaction speed is fast, and the reaction temperature is controlled at 50-60°C. As the reaction proceeds, the pH value of the reaction solution decreases. Therefore, in order to ensure the continuation of the reaction, the pH value of the reaction solution is controlled to be 8.5-10 by dropping 30% NaOH solution. Simultaneously, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Decomposition temperature | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com