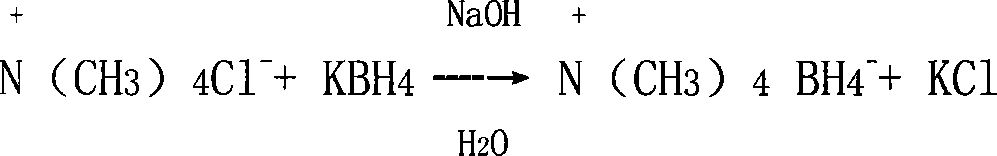Method for synthesizing L-2-amino propanol
A synthesis method and aminopropanol technology are applied in the preparation of aminohydroxy compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of difficulty in separation and purification, unsuitable for industrial production, etc., and achieve cost reduction and unit consumption reduction. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0011] 1. Preparation of reducing agent tetramethylammonium borohydride
[0012] Mix and stir 55.5g of tetramethylammonium chloride, 40g of sodium hydroxide, 0.5g of sodium chloride, and 100ml of water, slowly add 27.8g of potassium borohydride in 100ml of aqueous solution at room temperature for 30 minutes, and control the temperature at 35°C. Reaction 2h. After the reaction, 20-30ml of water was distilled off under reduced pressure, left to cool, and a large amount of tetramethylammonium borohydride crystals were formed, and filtered by suction to obtain 44.6g of tetramethylammonium borohydride, with a yield of 98.6%.
[0013] 2. Preparation of intermediate L-2-aminopropanol
[0014] (1) Esterification:
[0015] Mix and stir 18g of L-alanine and 300ml of ethanol, then cool down to below 4°C, add 26.5g of thionyl chloride dropwise within 30min, control the temperature not to exceed 5°C, remove the cooling, and react at 40°C for 4h. Evaporate about 250ml of ethanol from the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com