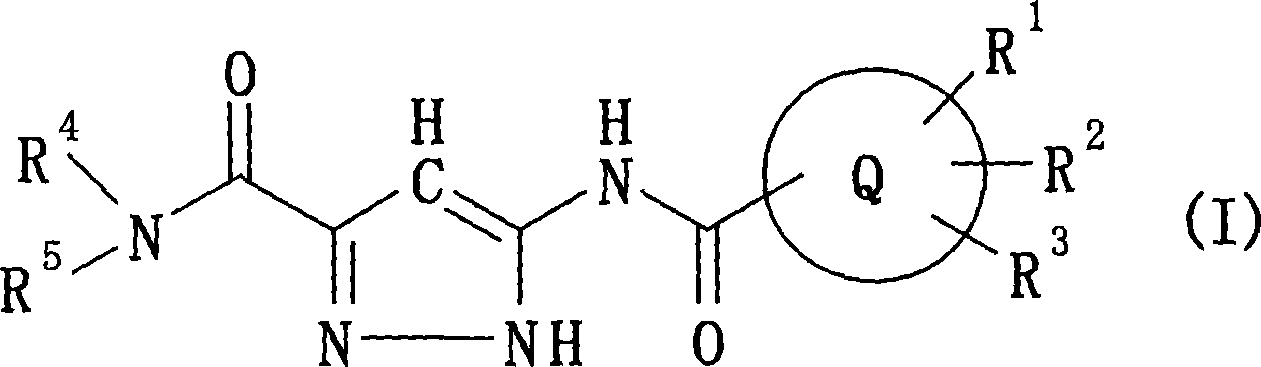
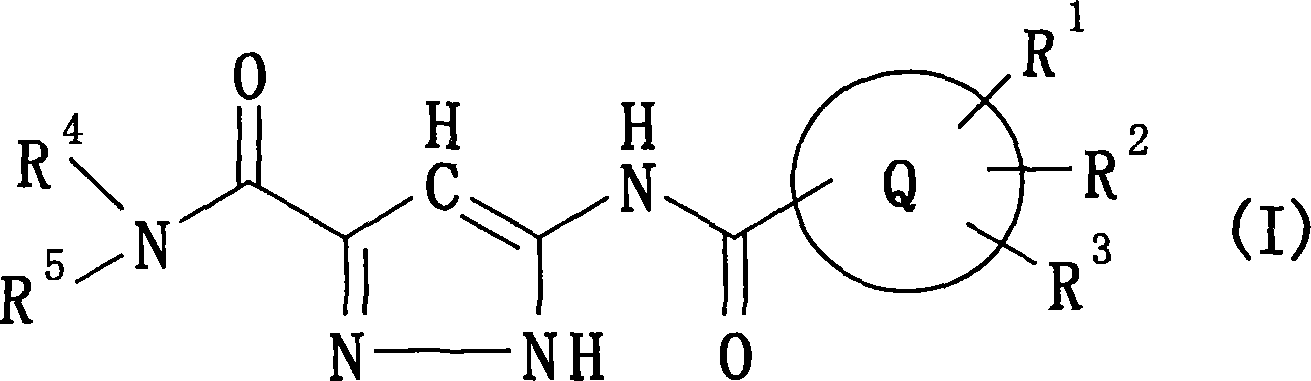
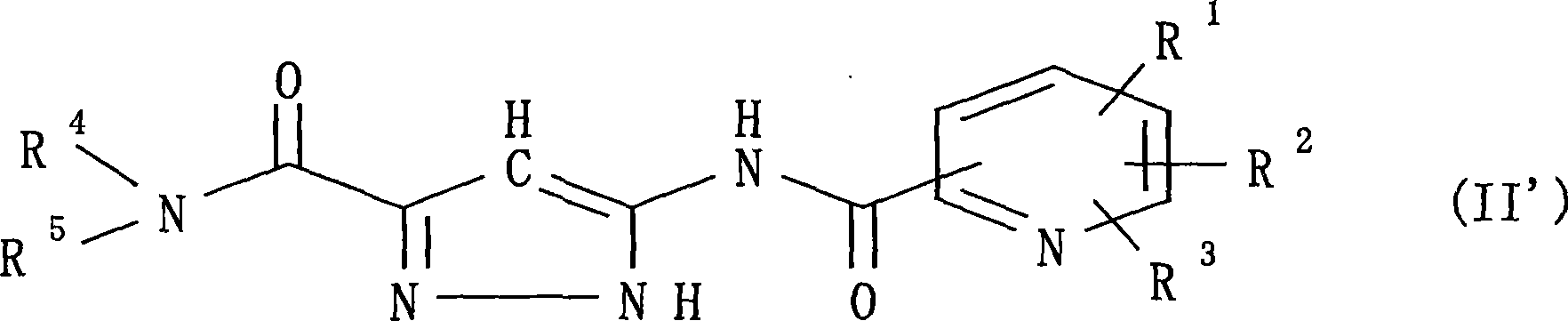
Pyrazole compounds and antidiabetes agents containing the same
A diabetes and compound technology, applied in the field of new pyrazole compounds, can solve problems such as unsatisfactory oral absorption and metabolic stability, and unsatisfactory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[2607] The production process scheme below is an example of a typical production method, but the production method of the present invention is not particularly limited to the following method. The compounds obtained in each step can be isolated and purified by conventional methods, but sometimes the next step can be carried out without isolation and purification.
[2608] Preparation method A (preparation methods A-1 to A-3):
[2609] Preparation method A is shown below. Each symbol in the reaction formula has the same meaning as above. The following are the same.
[2610]
[2611] Step 1; Preparation of Compound (A2)
[2612] The compound (A2) can be obtained by reducing the nitro group of the compound (A1) to an amino group with hydrogen in a solvent in the presence of a catalyst.
[2613]
[2614] As the solvent used in the reaction, for example, alcohol solvents such as methanol and ethanol; ether solvents such as dioxane and tetrahydrofuran; ester solvents su...
Embodiment
[2723] Hereinafter, the present invention will be described in detail by citing examples, but the present invention is not limited by these examples.
reference example 1
[2724] Reference Example 1: 5-(2-chloro-4,5-difluoro-benzoylamino)-1H-pyrazole-3-carboxylic acid imidazolide and 5-(2-chloro-4,5-difluoro-benzoylamino)-1H-pyrazole-3-carboxylic acid (benzene Preparation of triazol-1-yl)-ester;
[2725] (operation 1) preparation of 2-chloro-4,5-difluorobenzoyl chloride;
[2726]
[2727]In 2-chloro-4,5-difluorobenzoic acid (508.20g) in toluene (250ml) solution, add N,N-dimethylformamide (0.2ml), after heating to 65°C, add sulfite dropwise Acid chloride (230ml). After stirring at 120°C for 3 hours, the reaction solution was concentrated under reduced pressure, and the resulting residue was distilled under reduced pressure (bp 51-64°C (130-140Pa)) to obtain the title compound (534.45 g) as an oil.
[2728] (operation 2) preparation of ethyl 5-nitro-3-pyrazolecarboxylate;
[2729]
[2730] Methanesulfonic acid (143 ml) was added to a solution of 5-nitro-3-pyrazolecarboxylic acid (310.24 g) in ethanol (3 L), followed by stirring a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com