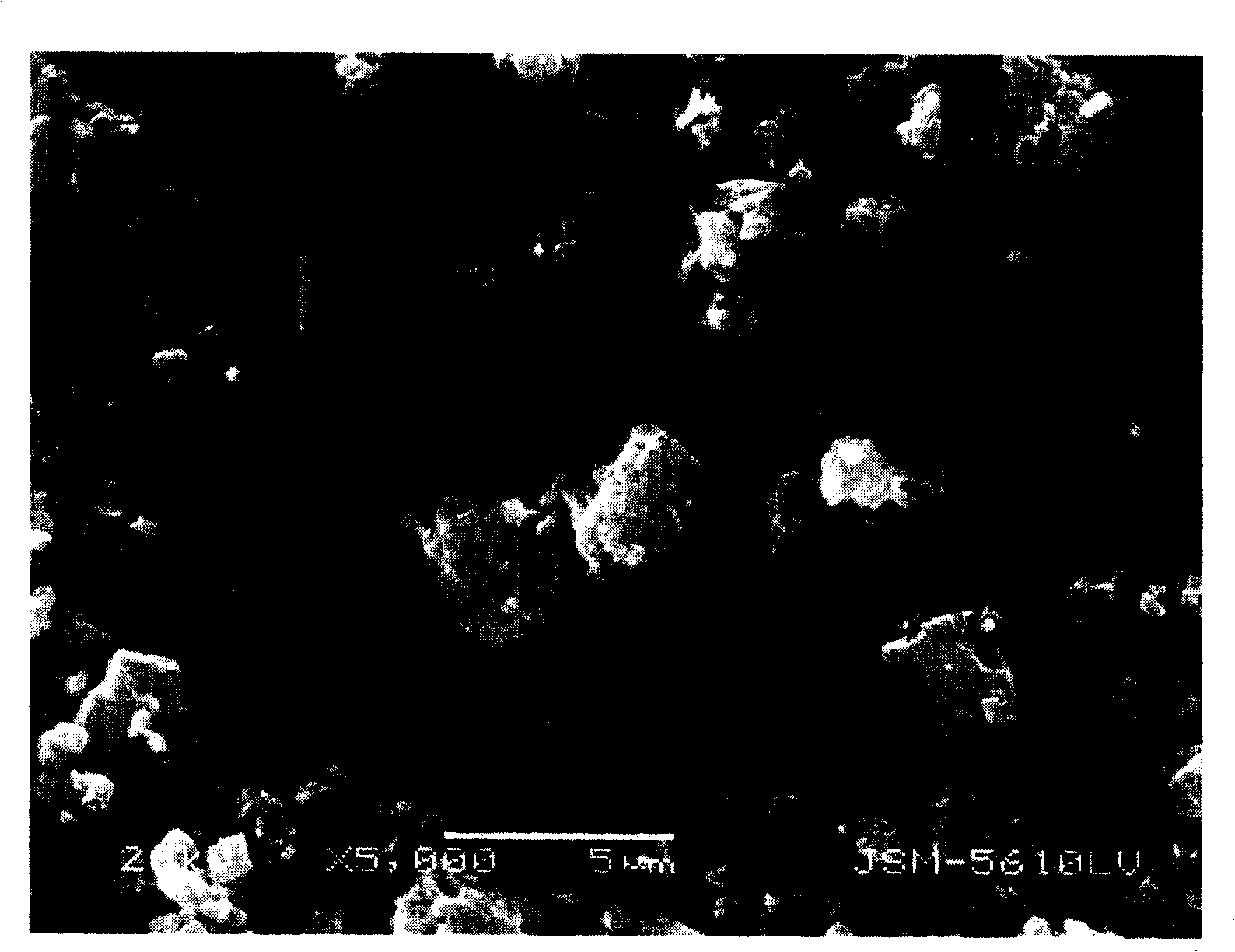
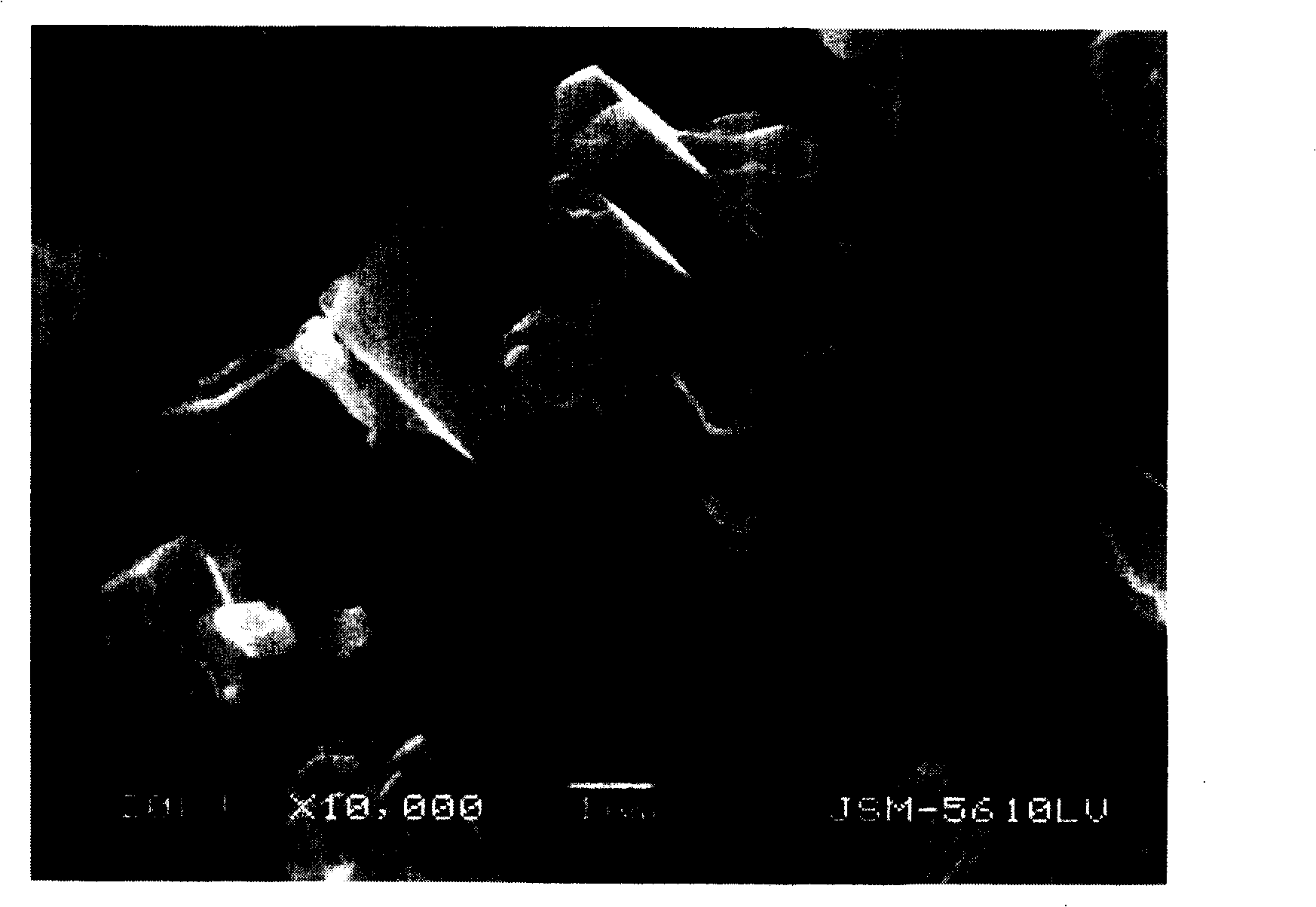
Method for preparing ferrous oxalate hydrated salt crystal
A technology of ferrous oxalate and oxalate, applied in the direction of organic chemistry, can solve the problems of large particle size and poor reactivity, and achieve the effect of good reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] The preparation method of the ferrous oxalate hydrate salt crystal provided by the present invention comprises the contact reaction of the oxalate aqueous solution and the ferrous salt solution, wherein, the contact reaction of the oxalate solution and the ferrous salt solution is carried out in an organic solvent and In the presence of a mixed solvent of water, the organic solvent is completely miscible with water.
[0020] According to the preparation method provided by the present invention, the contact reaction of the oxalate aqueous solution and the ferrous salt aqueous solution is to add the oxalate aqueous solution and the ferrous salt aqueous solution into a mixed solvent of an organic solvent and water.
[0021] According to the preparation method provided by the present invention, the pH value at the end of the reaction between the oxalate aqueous solution and the ferrous salt aqueous solution is preferably 1-5, more preferably 2-4. The pH value can be control...
Embodiment 1
[0037] 1.0 liter of ethanol (C 2 h 5 OH) is miscible with 0.2 liter of water to make a mixed solvent (temperature 25°C). Dissolve 556 g (2 moles) of ferrous sulfate heptahydrate (FeSO 4 ·7H 2 0), and add a small amount of sulfuric acid to adjust the pH value of the solution to 1.8. In addition, dissolve 368 grams (2 moles) of potassium oxalate monohydrate (K 2 C 2 o 4 ·H 2 O), and add a small amount of potassium hydroxide (KOH) aqueous solution to adjust the pH of the solution to 8.6. With a dropping speed of 50 ml / min, 2000 ml of potassium oxalate solution was dripped to this ethanol mixed solvent with 40 minutes, and at the same rate, 2000 ml of ferrous sulfate solution was dripped to this mixed solvent with 40 minutes. With the dropping of potassium oxalate solution and ferrous sulfate solution, ferrous oxalate precipitates (temperature 25°C, pH 2.8).
[0038] Next, the ferrous oxalate was recovered by filtration, and the recovered ferrous oxalate was washed with 1...
Embodiment 2
[0042] 335 ml ethanol (C 2 h 5 OH) was miscible with 65 ml of water to make a mixed solvent (temperature 25°C). Dissolve 556 grams (2 moles) of ferrous sulfate heptahydrate (FeSO 4 ·7H 2 O), and adding a small amount of sulfuric acid to adjust the pH value of the solution to 2.0. In addition, dissolve 368 grams (2 moles) of potassium oxalate monohydrate (K 2 C 2 o 4 ·H 2 O), and add a small amount of potassium hydroxide (KOH) aqueous solution to adjust the pH of the solution to 8.0. With a dropping speed of 100 ml / min, 2000 ml of potassium oxalate solution was dripped to this ethanol mixed solvent in 20 minutes, and at the same rate, 2500 ml of ferrous sulfate solution was dripped to this mixed solvent in 25 minutes. With the dropping of potassium oxalate solution and ferrous sulfate solution, ferrous oxalate precipitates (temperature 25°C, pH 2.5).
[0043] Next, the ferrous oxalate was recovered by filtration, and the recovered ferrous oxalate was washed with 10 lit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com