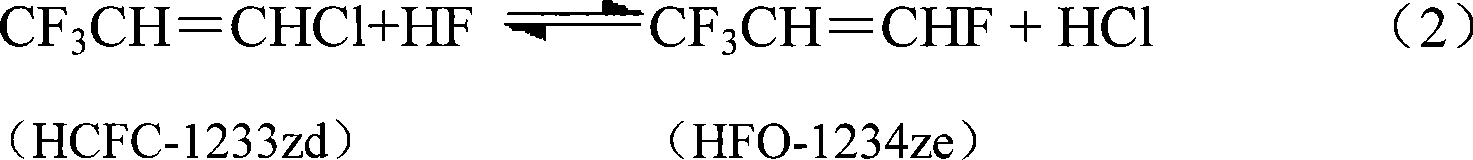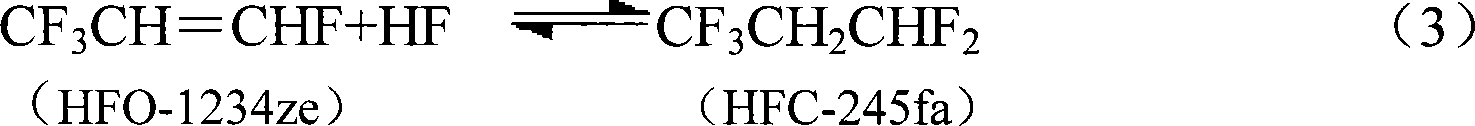Preparation method for 1,1,1,3-tetrafluoropropene
A technology of tetrafluoropropene and trifluoropropene, which is applied in halogen substitution preparation, dehydrohalogenation preparation and other directions, can solve the problems of difficult availability, low selectivity of HFO-1234ze, high price of raw material HCFC-1233zd, etc., and achieves improved selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
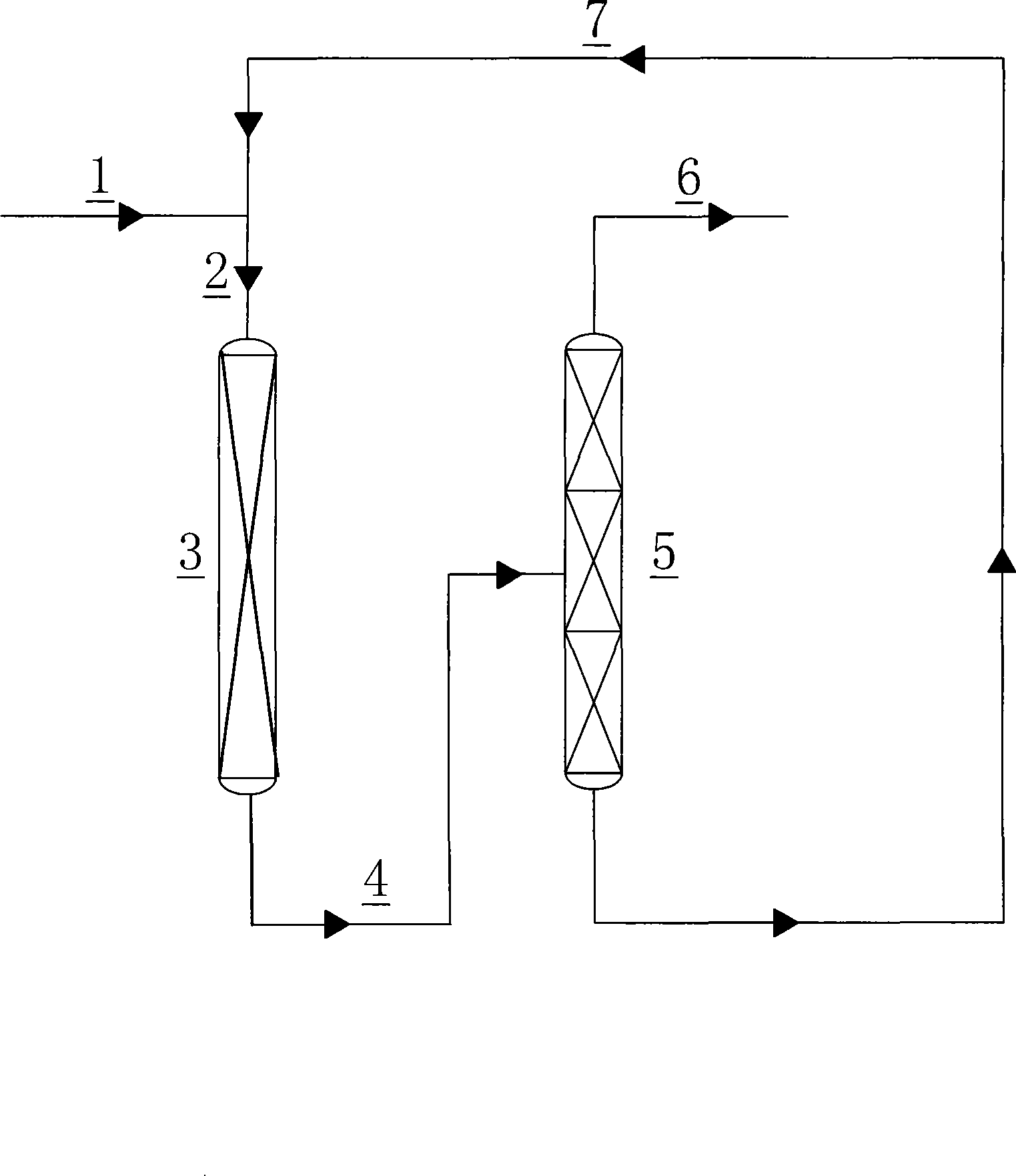
Image
Examples
Embodiment 1
[0030] In a nickel tube reactor with an inner diameter of 38 mm, add 50 ml of chromium-based fluorination catalyst containing Al, Zn, Mg, and Ni metal powders. HCC-240fa and HF enter the reactor to react. The reaction conditions are: the molar ratio of HF to HCC-240fa is 20, the reaction temperature is 300°C, the contact time is 7s, and the reaction pressure is 0.25Mpa. After 20 hours, the reaction product was washed with water, washed with alkali, and dried with alkali to remove HCl and HF, and then analyzed by gas chromatography. The reaction results are listed in Table 1.
Embodiment 2
[0032] The same operation as in Example 1, the difference is that the organic phase HCC-240fa at the reactor inlet is changed into a mixture of 40% by volume of HCC-240fa and 50% of HCFC-1233zd and 10% of HFC-245fa , and the results are listed in Table 1.
[0033] Table 1
[0034] Example
[0035] Other products include CF 3 CH 2 CHFCl (HCFC-244fa), CF 3 CH 2 CHCl 2 (HCFC-243fa), and traces of heavy substances C 6 h 4 f 7 Cl, C 6 h 4 f 6 Cl 2 、C 6 h 2 f 6 Cl 2 、C 6 h 3 f 6 Cl 3 Wait
Embodiment 3
[0037] In a nickel tube reactor with an inner diameter of 38 mm, add 50 ml of chromium-based fluorination catalyst containing Al, Zn, Mg, and Ni metal powders. The organic phase is composed of a mixture of 40% HCC-240fa and 55% HCFC-1233zd and 5% HFC-245fa by volume, and HF enters the reactor for reaction. The reaction conditions are: reaction temperature 280°C, total amount of HF and organic phase The molar ratio is 20, the contact time is 5s, and the reaction pressure is 0.02 Mpa. After 20h, the reaction product was washed with water, washed with alkali, and dried with alkali to remove HCl and HF, and then analyzed by gas chromatography. The reaction results are listed in Table 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com