Method for producing N-amido piperidine hydrochlorate
A technology of aminopiperidine and carbamoylpiperidine, which is applied in the field of preparation of N-aminopiperidine hydrochloride, can solve the problems of carcinogenicity of nitrosopiperidine and easy harm to the human body, and achieve easy post-processing, Easy operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
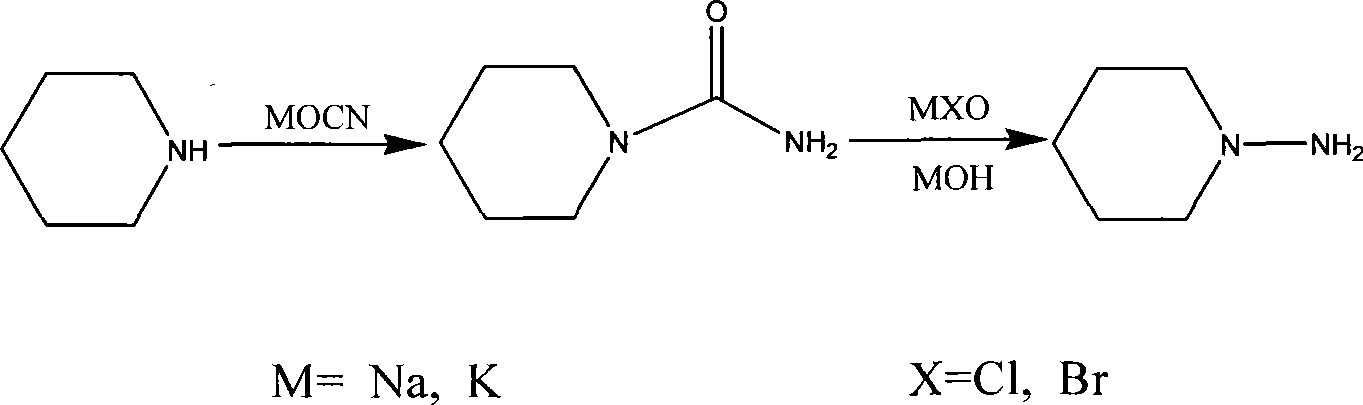
[0019] Dissolve 39.6 g of piperidine as a raw material in 40 ml of water, drop 50 ml of concentrated hydrochloric acid in an ice-water bath, and then add 40% sodium hydroxide solution to adjust the pH to 2.
[0020] Dissolve 31.5 g of sodium cyanate in 30 ml of water, add it into the above-mentioned piperidine solution at 10°C, and continue stirring at 20°C for 10 hours to obtain N-carbamoylpiperidine.
[0021] Cool the above-mentioned N-carbamoylpiperidine to -8°C, add aqueous sodium hydroxide solution, and adjust the pH to 13-14. After adding 386 grams of precooled sodium hypochlorite aqueous solution with a concentration of 10wt% under the same temperature conditions, react at room temperature for 15 hours, add sodium sulfite aqueous solution to destroy excess sodium hypochlorite, and after the aqueous layer is acidified with hydrochloric acid, concentrate and distill the water, using a volume of 1: 3 was recrystallized from ethanol-ethyl acetate to obtain 43.1 g of N-amino...
Embodiment 2
[0023] Dissolve 39.1 g of piperidine as a raw material in 40 ml of water, and add concentrated hydrochloric acid dropwise to adjust the pH to 4 under cooling in an ice-water bath.
[0024] Dissolve 34 grams of potassium cyanate in 56 ml of water, add to the above piperidine solution at 30°C, and continue stirring at 30°C for 8 hours to obtain N-carbamoylpiperidine.
[0025] The above-mentioned N-carbamoylpiperidine was cooled to 0°C, and an aqueous potassium hydroxide solution was added to adjust the pH to 13-14. After adding 586.8 grams of pre-cooled potassium hypobromite aqueous solution with a concentration of 12wt% under the same temperature condition, react at room temperature for 10 hours, add potassium sulfite aqueous solution to destroy excess potassium hypochlorite, after the aqueous layer is acidified with hydrochloric acid, concentrate and distill the water, use Recrystallization from ethanol-ethyl acetate with a volume ratio of 2:1 gave 34.9 g of N-aminopiperidine ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com