Multi-modification derivant of glutin and crosslinked material thereof
A technology of derivatives and gelatin, applied in the field of multiple modified derivatives of gelatin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
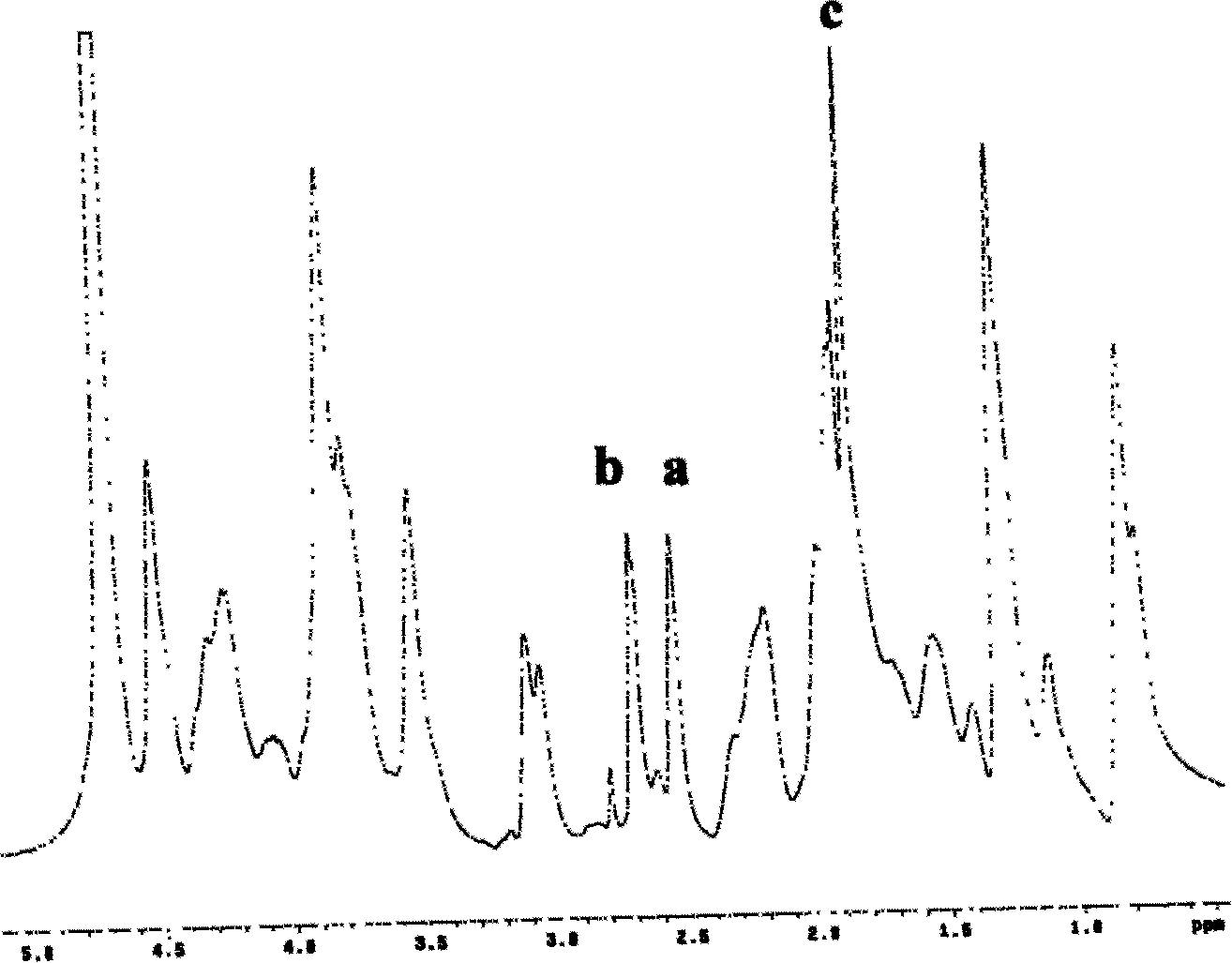
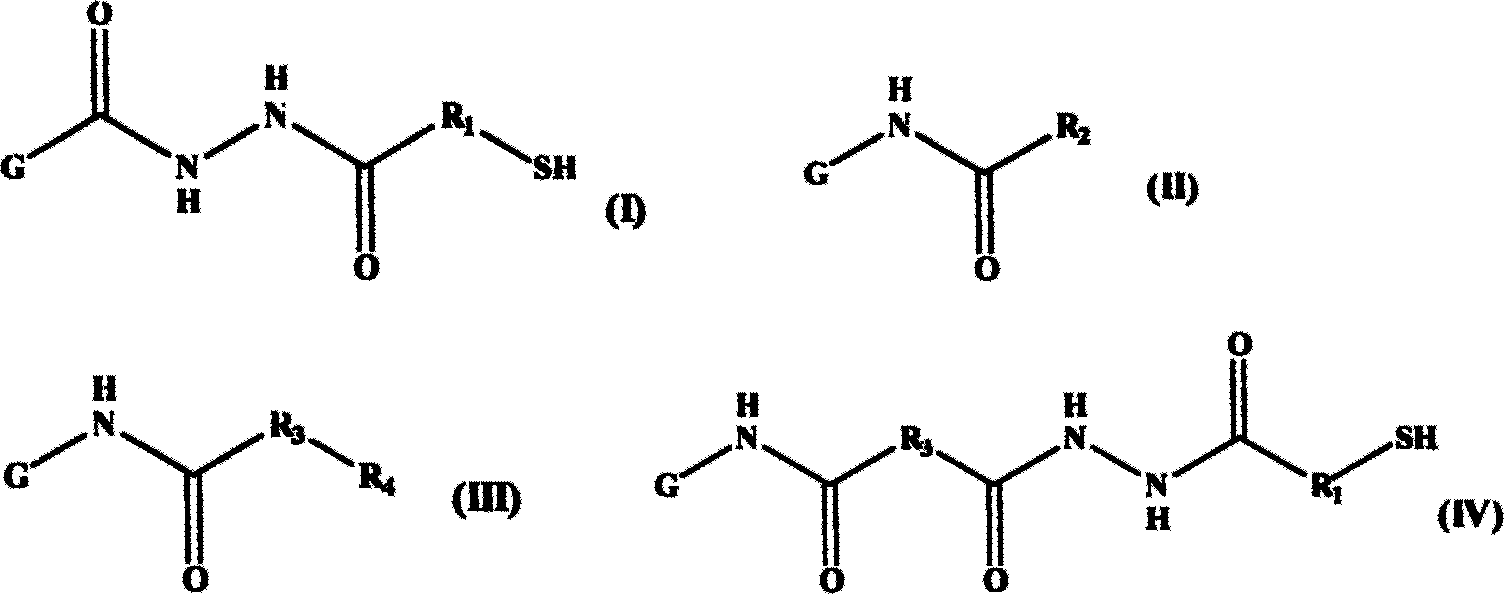
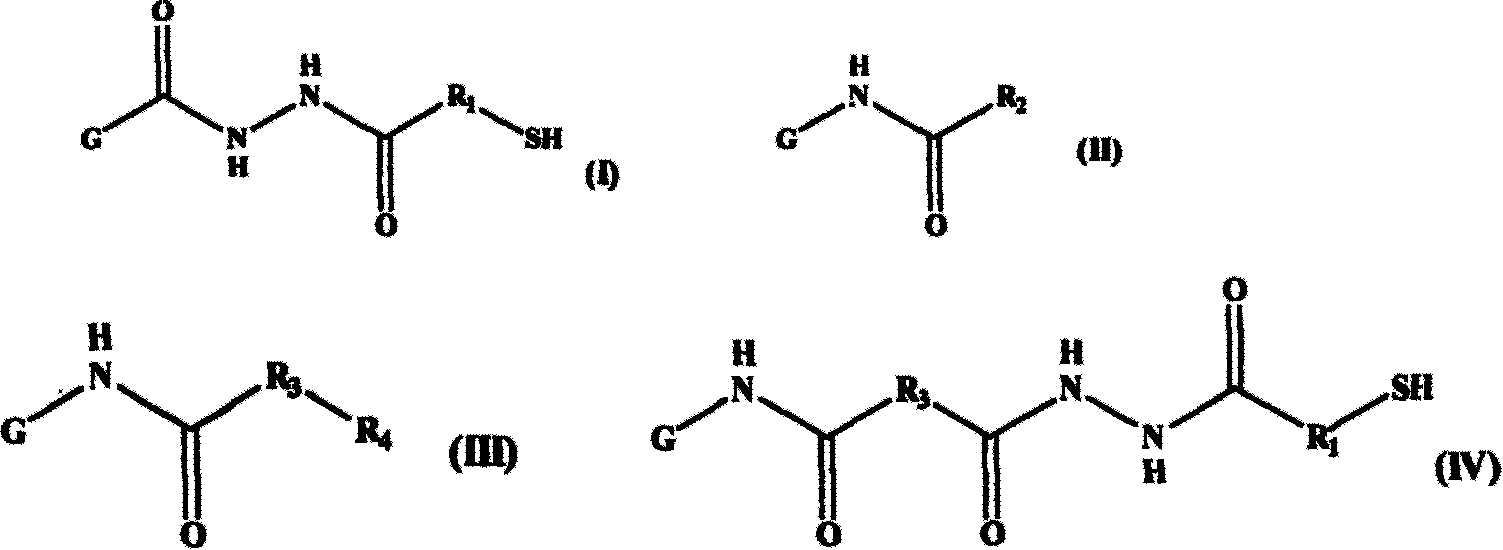
[0065] Example 1 Multiple modified derivatives of acetylated and thiolated gelatin (multiple modified derivatives of gelatin represented by general formula (V) of the present invention, wherein R 1 =-CH 2 CH 2 -, R 2 =-CH 3 ) Synthesis and characterization
[0066] (1) Acetylation modification of gelatin
[0067] One gram of gelatin (Type B, from Cowhide, Sigma, USA) was dissolved in 100 ml of distilled water (about 30 degrees Celsius) to obtain a clear and transparent solution. Use 1.0 mol / L sodium hydroxide solution to adjust the pH of the solution to about 9.5, then add 0.05 g of acetic anhydride (analytical purity) under electromagnetic stirring, and continue to add an appropriate amount of 1.0 mol / L sodium hydroxide solution to keep the solution The pH value is weakly alkaline (usually 8.0 ~ 9.5). The reaction was stirred at about 30 degrees Celsius for 1 hour. After that, the above solution was put into a dialysis tube (with a molecular weight cut off of 3500, Fisher, USA), ...
Embodiment 2
[0077] Example 2 Multiple modified derivatives of hexanoylated and thiolated gelatin (multiple modified derivatives of gelatin represented by general formula (V) of the present invention, wherein R 1 =-CH 2 CH 2 -, R 2 =-CH 2 CH 2 CH 2 CH 2 CH 3 ) Synthesis and characterization
[0078] (1) Caproacylation modification of gelatin
[0079] One gram of gelatin (type A, from pigskin, Sigma, USA) was dissolved in 100 ml of distilled water (about 30 degrees Celsius) to obtain a clear and transparent solution. Use 1.0 mol / L sodium hydroxide solution to adjust the pH of the solution to about 9.5, then add 0.1 g of valeric anhydride (analytical purity) under electromagnetic stirring, and continue to add an appropriate amount of 1.0 mol / L sodium hydroxide solution to keep the solution The pH value is weakly alkaline (usually 8.0 ~ 9.5). The reaction was stirred at about 30 degrees Celsius for 1 hour. After that, the above solution was put into a dialysis tube (with a molecular weight cut of...
Embodiment 3
[0090] Example 3 Multiple modified derivatives of butylated and thiolated gelatin (multiple modified derivatives of gelatin represented by general formula (V) of the present invention, wherein R 2 =-CH 2 CH 2 CH 3 ) Synthesis and characterization
[0091] (1) Butyryl modification of gelatin
[0092] One gram of gelatin (Type B, from Cowhide, Sigma, USA) was dissolved in 100 ml of distilled water (about 30 degrees Celsius) to obtain a clear and transparent solution. Adjust the pH of the solution to about 9.5 with 1.0 mol / L sodium hydroxide solution, then add 0.08 g of butyric anhydride (analytical pure) under electromagnetic stirring, and constantly add an appropriate amount of 1.0 mol / L sodium hydroxide solution to keep the solution The pH value is weakly alkaline (usually 8.0 ~ 9.5). The reaction was stirred at about 30 degrees Celsius for 1 hour. After that, the above solution was put into a dialysis tube (with a molecular weight cut off of 3500, Fisher, USA), dialyzed with dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com