Folic acid-polypeptide compound-mediated targeting anti-tumor prodrug and preparing method thereof
A technology of polypeptide complexes and prodrugs, which is applied in the direction of antineoplastic drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of no tumor prodrugs and achieve good application and promotion value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
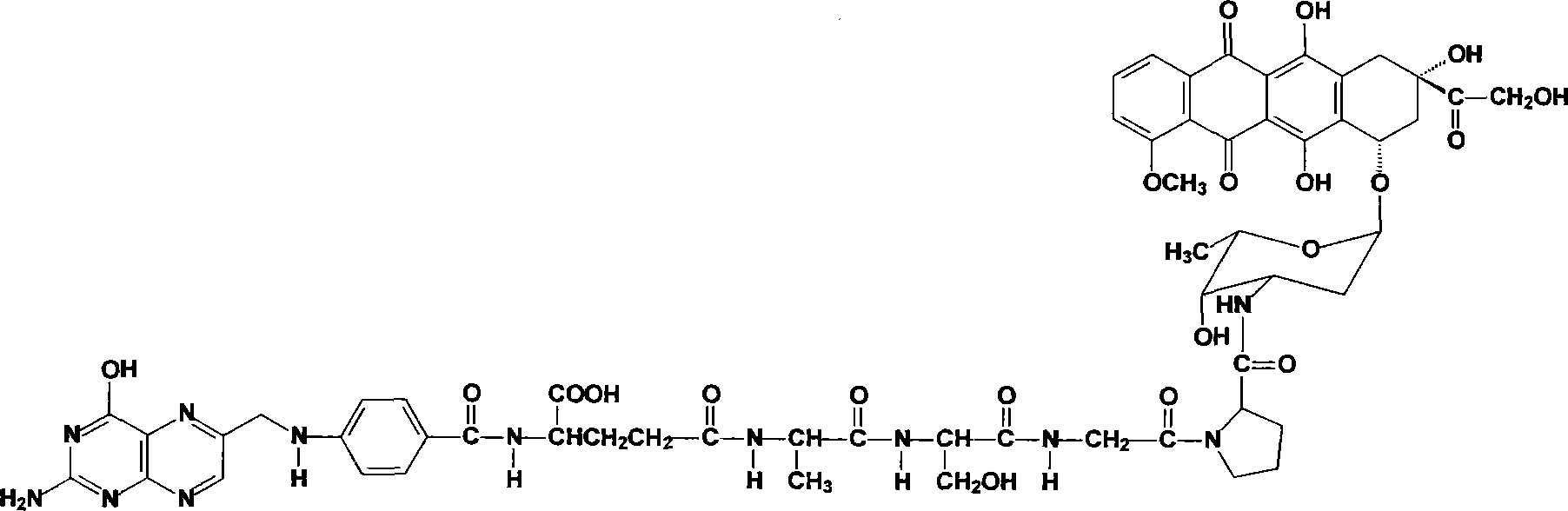
[0038] Example 1 Synthesis of folic acid-alanylserylglycylprolyl-doxorubicin (F-ASGP-Dox)
[0039] (1) Synthesize prolyl-resin, measure the loading value of prolyl-resin; synthesize polypeptide-resin;
[0040] A. Synthesis of prolyl-resin
[0041] Weigh 300 mg of 2-chloro-trityl chloride resin (1.0 mmol / g), wash with DCM 3 times, soak in DCM for 5 min, and dry under N2 pressure. Add DCM to 304 mg of Fmoc-proline and 128 mg of DIPEA dropwise until it is completely dissolved, add the mixture to the resin, stir and react for 5 minutes, then add 225 mg of DIPEA, react for 2 to 3 hours, then add 300 μl of methanol, After 10 minutes of reaction, the measured loading value was 0.8. Add 1.0 ml of 25% piperidine / DMF to deprotect the Fmoc group. DMF was washed 5 times, and N2 was pressed to dry.
[0042] B, Synthesis of alanylserylglycylprolyl-resin
[0043] Weigh 147 mg of Fmoc-glycine, 285 mg of HOBt, and 121 mg of DIC. After dissolving DMF, add prolyl-resin. After reacting for 2...
Embodiment 2
[0050] Example 2 Synthesis of folic acid-alanylserylglycylprolyl-mephalan (F-ASGP-PAM)
[0051] (1) Synthesize F-ASGP according to the steps (1) and (2) of Example 1
[0052] (2) Take N folic acid-alanyl seryl glycyl proline and dissolve it in N,N-dimethylformamide, add the anti-tumor cytotoxic drug solution dissolved in Et3N DMF after activation, stir and react Afterwards, diethyl ether was added for extraction, and the diethyl ether extract was subjected to rotary evaporation to obtain the crude product of folic acid-polypeptide-melphalan.
[0053] Weigh 5 mg of F-ASGP and dissolve it in 1 ml of N,N-dimethylformamide (DMF), add 2 μl of ethyl chloroformate, and stir at room temperature for 2 minutes. Add 2.8 μl triethylamine and stir at room temperature for 1 hour.
[0054] Weigh 2 mg of phenylalanine mustard (PAM) and dissolve it in 0.3 mL of DMF, add 1 μl of triethylamine to it and mix well. Add the dissolved melphalan into the activated F-ASGP solution, and stir at room...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com