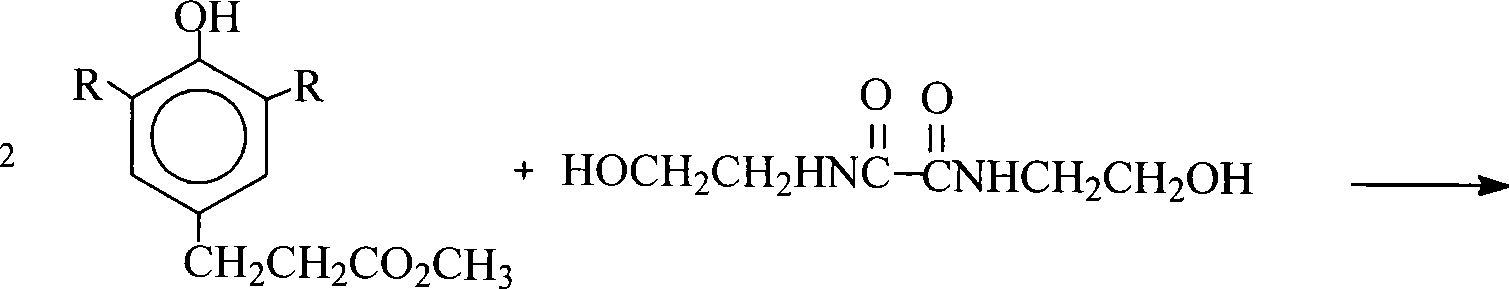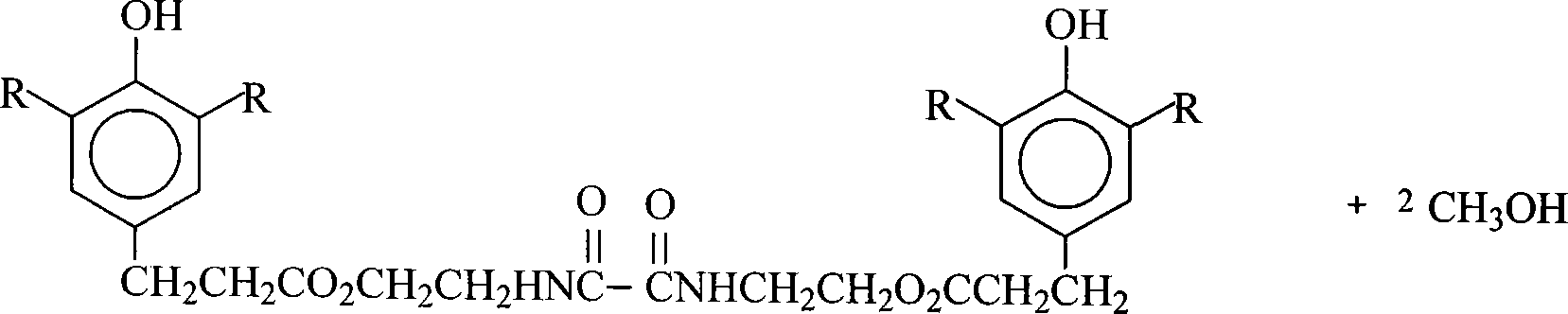Preparation method of anti-oxidizing agent
An anti-oxidant and solvent technology, applied in the field of N, can solve the problems of conversion rate and selectivity that are difficult to reach the theoretical level, waste of raw materials, environmental pollution, etc., and achieve the effects of saving raw material consumption, increasing yield, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add 50ml of xylene to a 250ml four-necked flask equipped with a stirrer, a thermometer, a nitrogen conduit, a water trap and a condenser, start stirring, and add 17.6g of N,N'-bis(2-hydroxyethyl)oxamide ( 0.10mol), 3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionic acid 61.2g (0.22mol), monobutyl stannoic acid 0.4g (0.0019mol), feed nitrogen, be heated to reflux, keep The temperature of the reaction solution is about 160°C, and the reaction is refluxed for 6.0 hours, and the amount of dehydration is 3.5-3.6g. Cool down to 100°C, add 2.0g of activated carbon, keep stirring at 120-130°C for 30 minutes, cool down to 80-100°C, and filter. Remove the xylene reaction solvent by distilling the reaction solution at 180°C under normal pressure and under reduced pressure (20mmHg), cool it down to 75-80°C, add 200ml of isopropanol to crystallize the material, cool it down to room temperature, filter through a Buchner funnel, and use isopropanol Rinse with alcohol and dry to obtain 6...
Embodiment 2
[0017] The process and conditions of Example 1 were repeated, and xylene was replaced with toluene, and the amount of toluene was 35ml. Obtained 60.1 g of N,N'-bis(2-hydroxyethyl)oxalamide-bis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate, melting point 172-174°C, purity 98.8%, yield 86.23% [calculated by N, N'-bis(2-hydroxyethyl)oxamide]. Reclaim 3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionic acid 10.4g, final product yield 94.38% [according to 3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionic acid meter].
Embodiment 3
[0019] Repeat the technique and condition of embodiment 1, decolorizing agent changes gac into activated clay, isopropanol is replaced as ethanol. Obtained 61.1 g of N,N'-bis(2-hydroxyethyl)oxalamide-bis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate, melting point 172-174°C, purity 98.7%, yield 87.66% [calculated by N, N'-bis(2-hydroxyethyl)oxamide]. Reclaim 3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionic acid 10.7g, final product yield 96.51% [according to 3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionic acid meter].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com