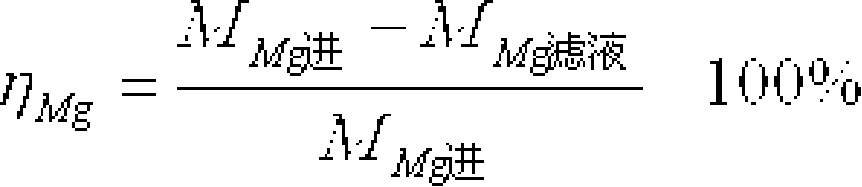Technique for preparing high purity ultra-fine magnesium hydroxide by sodium hydroxide method
A technology of ultra-fine magnesium hydroxide and sodium hydroxide, applied in the direction of magnesium hydroxide, etc., can solve problems such as difficulty in washing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
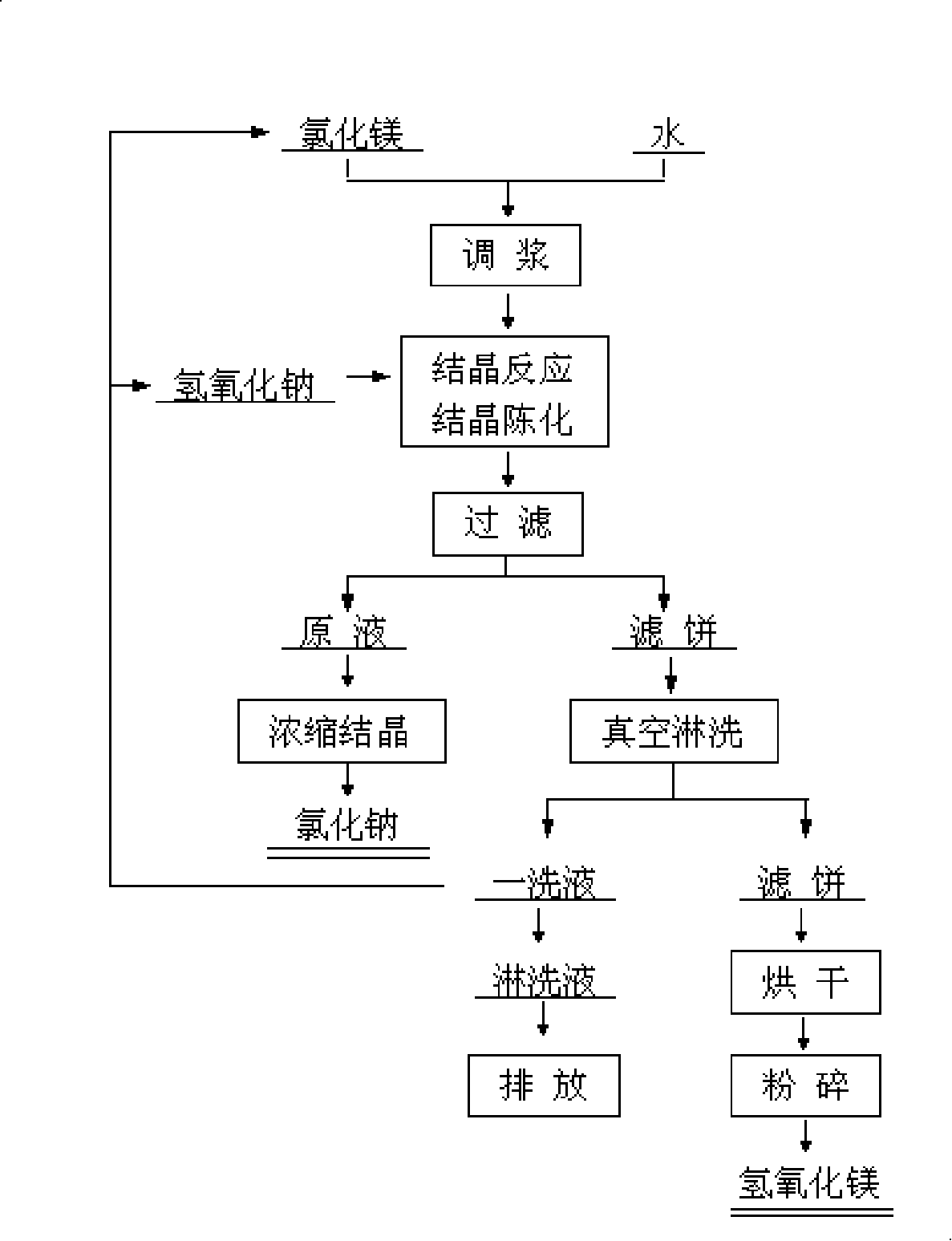
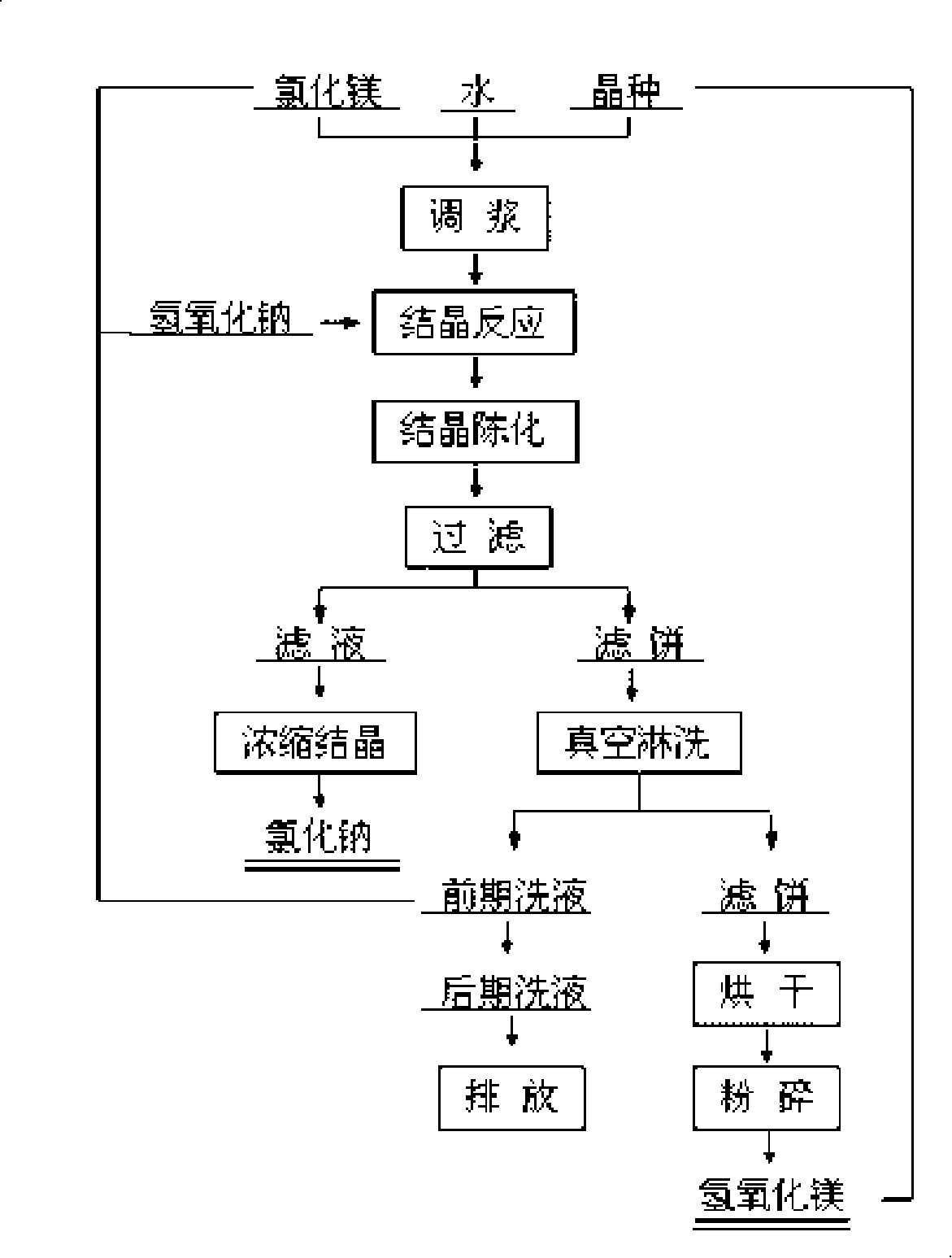
[0134] The process of producing high-purity ultra-fine magnesium hydroxide by sodium hydroxide dry crystal seed decomposition method is as follows: Mg 2+ Purified brine with a concentration of 20g / L and solid magnesium hydroxide seed crystals are added to the reaction tank and mixed, and kept at a certain temperature (60-80°C), the seed crystal coefficient is 1.0, and then sodium hydroxide solution (350g / l) is added to the reaction tank Precipitation reaction was carried out in the medium, the alkali excess coefficient was 1.05, crystallization and aging at a temperature of 70°C for 90 minutes, after the reaction was completed, the magnesium hydroxide slurry was extracted and filtered, and washed several times (liquid-solid ratio L / S=8, washing temperature 40°C, Washing time is 10 minutes), the filtered slurry stock solution is used to evaporate and extract chemically pure sodium chloride; the washing liquid is returned to the process for batching or system replenishment. The ...
Embodiment 2
[0136] The process of producing high-purity ultra-fine magnesium hydroxide by sodium hydroxide wet seed circular decomposition method is as follows: Mg 2+ The purified brine with a concentration of 30g / L and the magnesium hydroxide slurry obtained from the previous reaction are mixed in the reaction tank, kept at a certain temperature (60-80°C), and the seed crystal coefficient is 0.5, and then the sodium hydroxide solution (300g / l) Put it into the reaction tank for precipitation reaction, the alkali excess coefficient is 1.01, crystallize and age at 55°C for 120 minutes, after the reaction is completed, take part of the magnesium hydroxide slurry for filtration and continuous washing, and the filtered slurry stock solution is used for evaporation and extraction of chemical Pure sodium chloride; the washing liquid is used for batching or system water replenishment in the return process; the final eluent can be directly discharged or returned to the system as water replenishment...
Embodiment 3
[0138] The process of producing high-purity ultra-fine magnesium hydroxide by sodium hydroxide wet seed circular decomposition method is as follows: Mg 2+ The purified brine with a concentration of 25g / L and the magnesium hydroxide slurry obtained from the previous reaction are mixed in the reaction tank, kept at a certain temperature (60-80°C), and the seed crystal coefficient is 1.5, and then the sodium hydroxide solution (320g / l) Add it into the reaction tank for precipitation reaction, the alkali excess coefficient is 1.03, and crystallize and age at 90°C for 30 minutes. After the reaction, take part of the magnesium hydroxide slurry for filtration, continuous vacuum filtration and rinsing, and use the filtered slurry stock solution It is used to extract chemically pure sodium chloride by evaporation; it is used for batching or system replenishment in the washing liquid return process; finally, the eluent can be directly discharged or returned to the system as replenishing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com