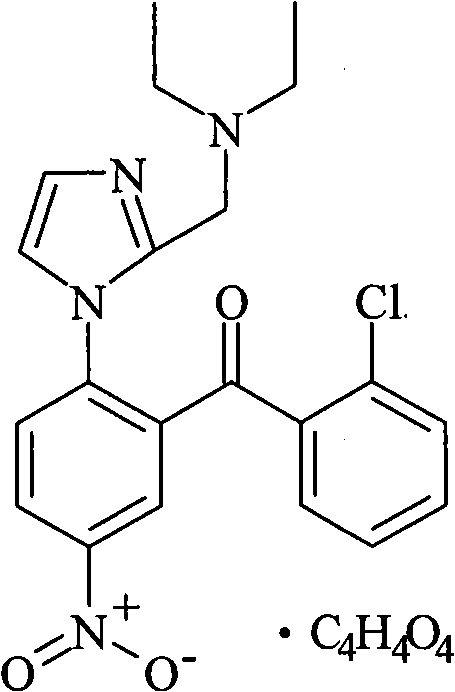
Boletic acid buddhist nun azoles phenyl ketone freeze-dried powder and method of preparing the same
A technology of nizophenone fumarate and freeze-dried powder, which is applied in the field of medicine, can solve the problems of poor solubility of microcrystalline cellulose, narrow application range, easy decomposition and deterioration, etc., and achieves easy large-scale industrial production and simple preparation method Easy-to-use, wide-ranging effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Nizophenone Fumarate 5g
[0052] Mannitol 3g
[0053] Tween 80 2g
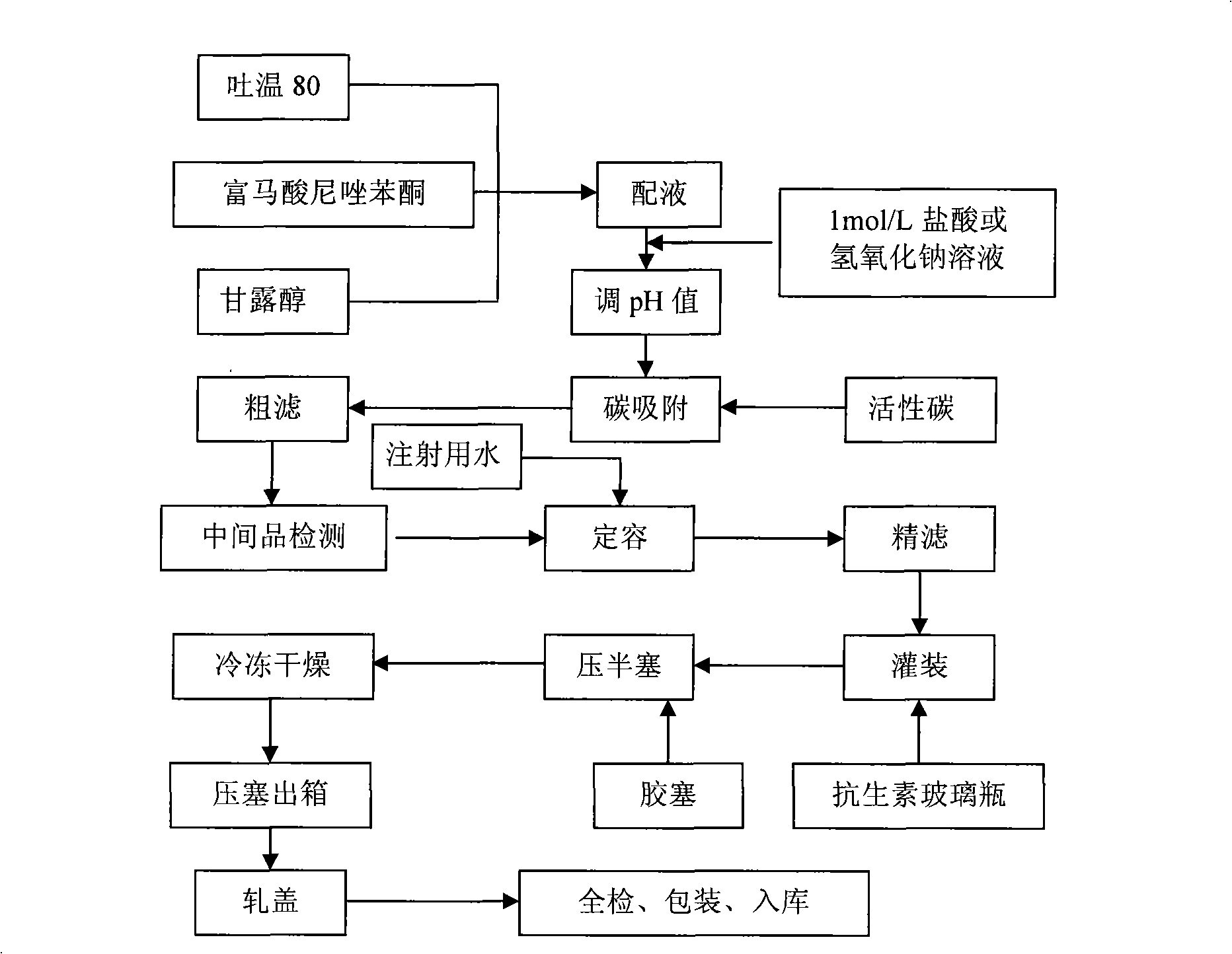
[0054] Put the main and auxiliary materials into the liquid mixing tank, add 1200m water for injection to dissolve, adjust the pH to 3~4 with hydrochloric acid or sodium hydroxide, then add 0.05% medical activated carbon and stir for 20 minutes to decolorize, then filter to remove carbon, and the filtrate is qualified after pH measurement Add water for injection until the volume of the solution is 2000ml, fine filter with a 0.22μm microporous membrane, subpackage the filtrate, and half-tamp the plug, freeze the subpackaged drug solution to -45°C, keep it frozen for 3 hours, and then evacuate to 1mmHg, the temperature was raised slowly at a constant speed of 3°C / hour to 15°C, and then raised to 45°C at a speed of 6°C / hour, then dried at a constant temperature for 5 hours, capped, packed and put into storage.
Embodiment 2
[0056] Nizophenone Fumarate 5g
[0057] Dextran 2.5g
[0058] Hydroxypropyl beta cyclodextrin 2g
[0059] Put the main and auxiliary materials into the liquid mixing tank, add 1200ml of water for injection to dissolve, adjust the pH to 3-11 with hydrochloric acid or sodium hydroxide, then add 0.05% medical activated carbon and stir for 10 minutes to decolorize, then filter to remove carbon, and the filtrate is qualified after pH measurement Add water for injection until the volume of the solution is 2000ml, fine filter with a 0.22μm microporous membrane, subpackage the filtrate, and half-tamp the plug, freeze the subpackaged drug solution to -60°C, insulate and freeze for 1 hour, and then evacuate to 10mmHg, the temperature was raised slowly at a constant speed of 5°C / hour to 0°C, and then the temperature was raised to 50°C at a speed of 9°C / hour, then dried at a constant temperature for 3 hours, capped, packed and put into storage.
Embodiment 3
[0061] Nizophenone Fumarate 5g
[0062] Dextran 50g
[0063] Poloxamer 2g
[0064] Put the main and auxiliary materials into the liquid mixing tank, add 1200ml of water for injection to dissolve, adjust the pH to 5-7 with hydrochloric acid or sodium hydroxide, then add 0.05% medical activated carbon and stir for 30 minutes to decolorize, then filter to remove carbon, and the filtrate is qualified after pH measurement Add water for injection until the volume of the solution is 2000ml, fine filter with a 0.22μm microporous membrane, subpackage the filtrate, and half-tamp the plug, freeze the subpackaged drug solution to -30°C, keep it frozen for 6 hours, and then evacuate to 0.5mmHg, the temperature was raised slowly at a constant speed of 2°C / hour to 0°C, and then raised to 40°C at a speed of 7°C / hour, and then dried at a constant temperature for 7 hours, capped, packed and put into storage.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com