Method for preparing ferric lithium phosphate precursor by comprehensive utilization of ilmenite
A lithium iron phosphate and precursor technology is applied in the field of comprehensively utilizing ilmenite to prepare lithium iron phosphate precursors for cathode materials of lithium ion batteries, and achieves the effects of simple process flow, stable product quality and good product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
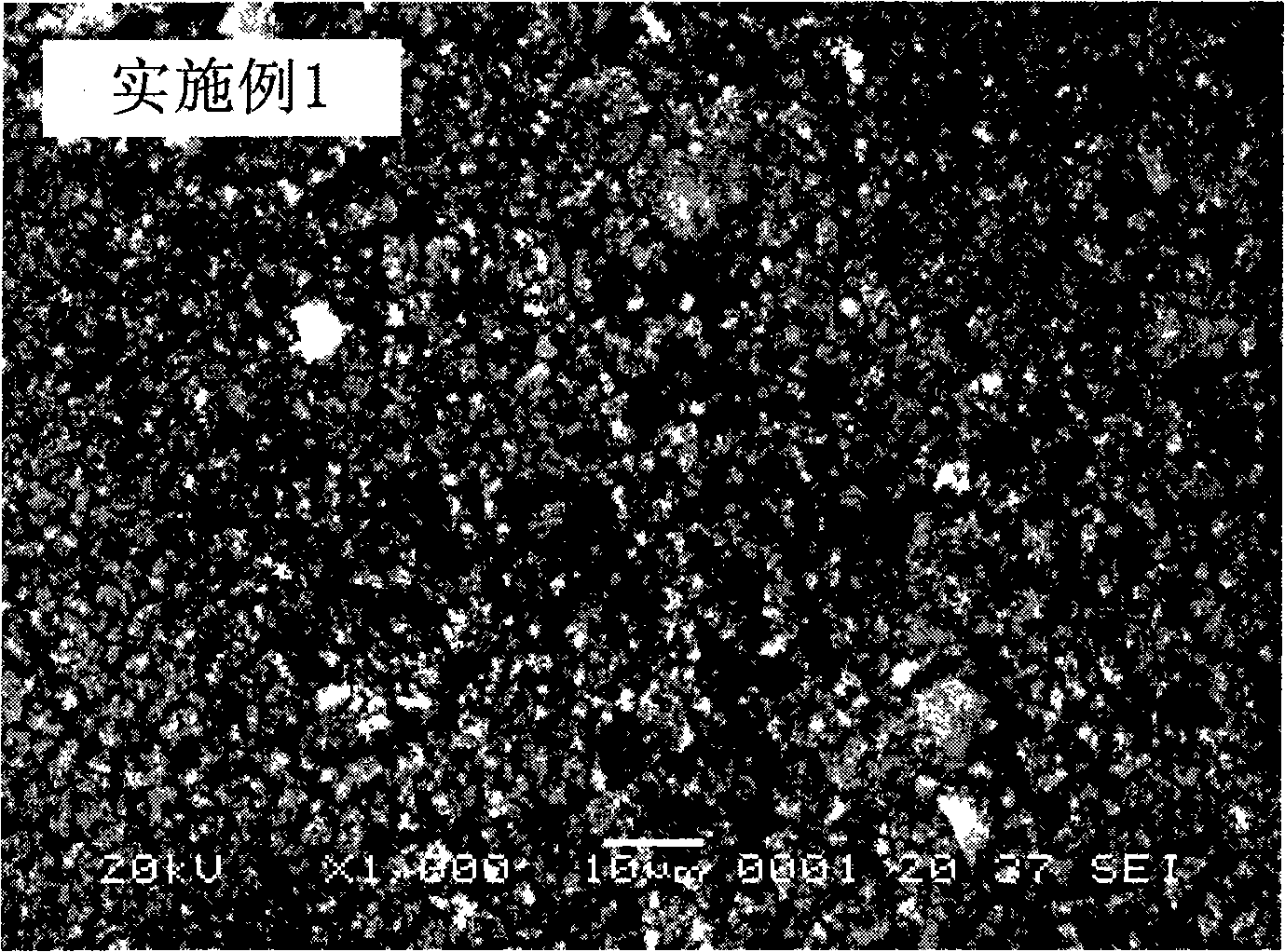
Embodiment 1
[0027] Leach 1kg of ilmenite with sulfuric acid, filter to obtain filtrate, dissolve a certain amount of hematite and ferric sulfate in the filtrate, so that the concentration of Fe in the mixed solution is 0.1mol / L, and the molar ratio of Ti to Fe is 0.5; Add an appropriate amount of 0.01mol / L sodium peroxide solution to the mixed solution, adjust the pH of the system to about 1.5 with 0.5mol / L sodium hydroxide solution, stir for a few minutes and filter to obtain the filtrate; add an appropriate amount of 1mol / L ammonium carbonate solution, adjust the pH of the system to about 6.0 with 2mol / L ammonia water, react in a stirred reactor at 50°C for 10min, filter, wash, dry the precipitate at 100°C and put it in the air Calcining at 600° C. for 2 hours to obtain the precursor of lithium iron phosphate, the anode material of the lithium ion battery, and the ferric oxide of the doped metal element.
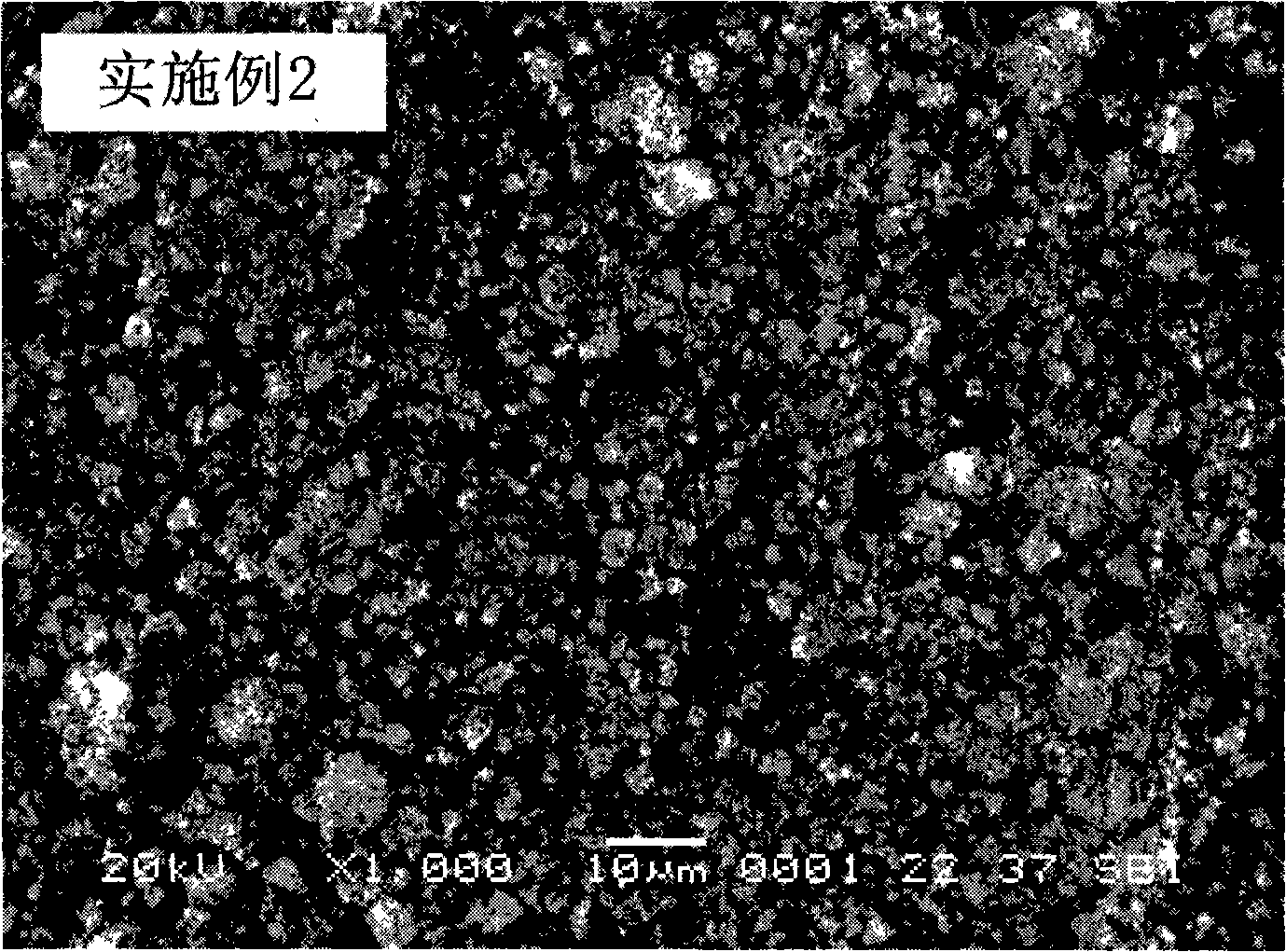
Embodiment 2
[0029] Leach 1 kg of ilmenite with hydrochloric acid, filter to obtain the filtrate, dissolve a certain amount of ferric chloride in the filtrate, so that the concentration of Fe in the mixed solution is 1mol / L, and the molar ratio of Ti to Fe is 0.3; Add an appropriate amount of 3mol / L sodium hypochlorite solution, adjust the pH of the system to about 3.0 with a 2mol / L lithium hydroxide solution, and filter after stirring for several minutes to obtain a filtrate; add an appropriate amount of 2mol / L sodium carbonate solution in the filtrate, and use 0.1mol / L sodium hydroxide solution to adjust the pH of the system to about 4.0, react in a stirred reactor at 10°C for 4h, filter and wash, dry the precipitate at 50°C, and then calcinate at 800°C in air for 1h That is, the precursor of the positive electrode material lithium iron phosphate of the lithium ion battery-the ferric oxide of the doped metal element is obtained.
Embodiment 3
[0031] Leach 1 kg of ilmenite with sulfuric acid, filter to obtain the filtrate, dissolve a certain amount of ferrous sulfate and ferrous chloride in the filtrate, so that the concentration of Fe in the mixed solution is 2mol / L, and the molar ratio of Ti to Fe is 0.1 Add an appropriate amount of 1mol / L potassium chlorate solution to the mixed solution, adjust the pH of the system to about 6.0 with 3mol / L ammonia water, filter after stirring for several minutes, and obtain the filtrate; add an appropriate amount of 3mol / L potassium bicarbonate to the filtrate Solution, use 6mol / L potassium hydroxide solution to adjust the pH of the system to about 14.0, react in a stirred reactor at 70°C for 2h, filter and wash, dry the precipitate at 150°C, and then calcinate in air at 300°C After 24 hours, the precursor of lithium iron phosphate, the cathode material of the lithium ion battery, is obtained—the ferric oxide of the doped metal element.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com