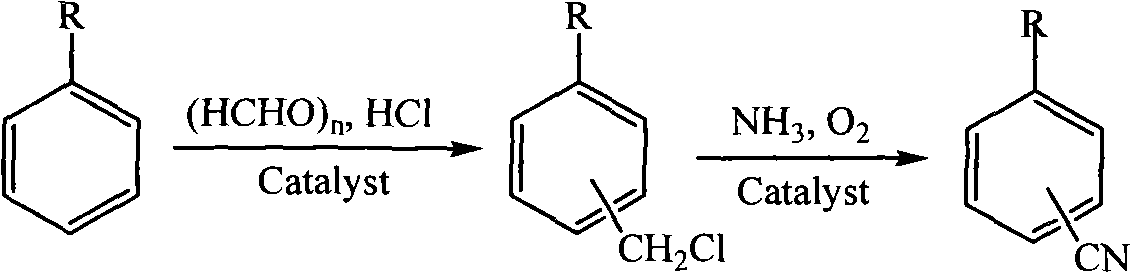
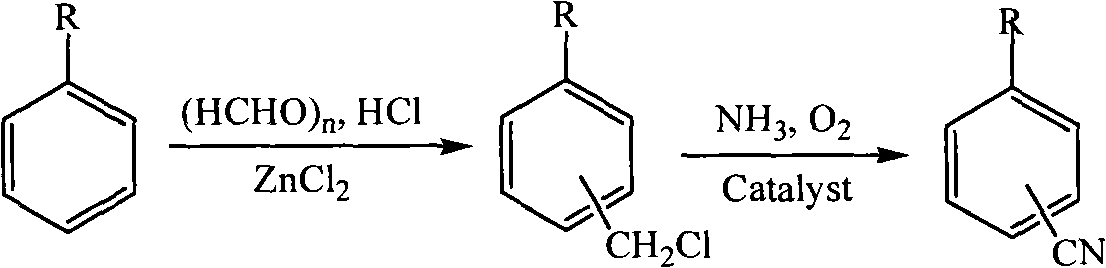
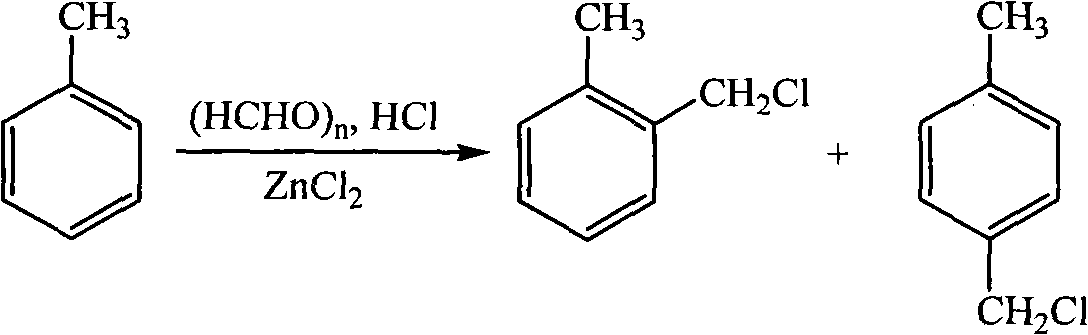
Method for preparing alkylcyanophenyl
A technology for alkyl benzonitrile and alkyl benzene is applied in the field of preparing alkyl benzonitrile, and can solve the problem of low dialkyl benzene conversion rate, low product yield, difficult alkyl benzonitrile synthesis reaction, and tert-butyl benzonitrile selectivity. low problems, to achieve the effect of good application value, simple preparation method and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] Add 1.5015g of paraformaldehyde, 3.4075g of anhydrous zinc chloride and 7.5ml of concentrated hydrochloric acid into a 250ml three-necked flask, then heat and stir to fully mix the solid zinc chloride and paraformaldehyde with concentrated hydrochloric acid, when the temperature rises to 60°C 19.8 g of toluene was added, and HCl gas was introduced under vigorous stirring for 6 h. After the reaction was finished, the product was transferred to a separatory funnel for static layering, and the concentrated hydrochloric acid solution of the lower floor was first separated, and then the solution was washed with 10% Na 2 CO 3 Aqueous and distilled water washes, anhydrous Na 2 SO 4 After drying, use a rotary evaporator to evaporate excess toluene, and finally distill under reduced pressure to obtain a mixture of o-methylbenzyl chloride and p-methylbenzyl chloride with a yield of 86.5%.
Embodiment 2
[0026] Add 1.5 g of paraformaldehyde, 2.0 g of anhydrous aluminum chloride and 20 ml of ethylbenzene into the three-neck flask, raise the temperature to 60° C., and pass HCl gas under vigorous stirring for 8 h. After the reaction was over, the product was transferred to a separatory funnel and washed with 10% Na 2 CO 3 Aqueous and distilled water washes, anhydrous Na 2 SO 4 After drying, use a rotary evaporator to evaporate excess ethylbenzene, and finally distill under reduced pressure to obtain a mixture of o-ethylbenzyl chloride and p-ethylbenzyl chloride with a yield of 89.2%.
Embodiment 3
[0028] Add 2.0g of formaldehyde, 3ml of concentrated sulfuric acid, 6ml of concentrated hydrochloric acid and 20ml of cumene into the three-neck flask respectively, then heat to 40°C, and inject HCl gas for 5h under vigorous stirring. After the reaction was finished, the product was transferred to a separatory funnel for static layering, and the concentrated hydrochloric acid solution of the lower floor was first separated, and then the solution was washed with 10% Na 2 CO 3 Aqueous and distilled water washes, anhydrous Na 2 SO 4 After drying, excess cumene was distilled off first by vacuum distillation, and then the mixture of o-cumyl benzyl chloride and p-cumyl benzyl chloride was obtained by vacuum distillation with a yield of 78.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com