Method for synthesizing rosin or rosin derivatives methacrylic ester
A technology of methallyl ester and rosin derivatives, which is applied in the field of rosin derivatives synthesis, can solve the problems of easy thermal polymerization, cannot use esterification conditions, etc., and achieves the effects of reduced dosage, high production efficiency and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 A kind of synthetic method of rosin or rosin derivative methallyl ester comprises the following steps,
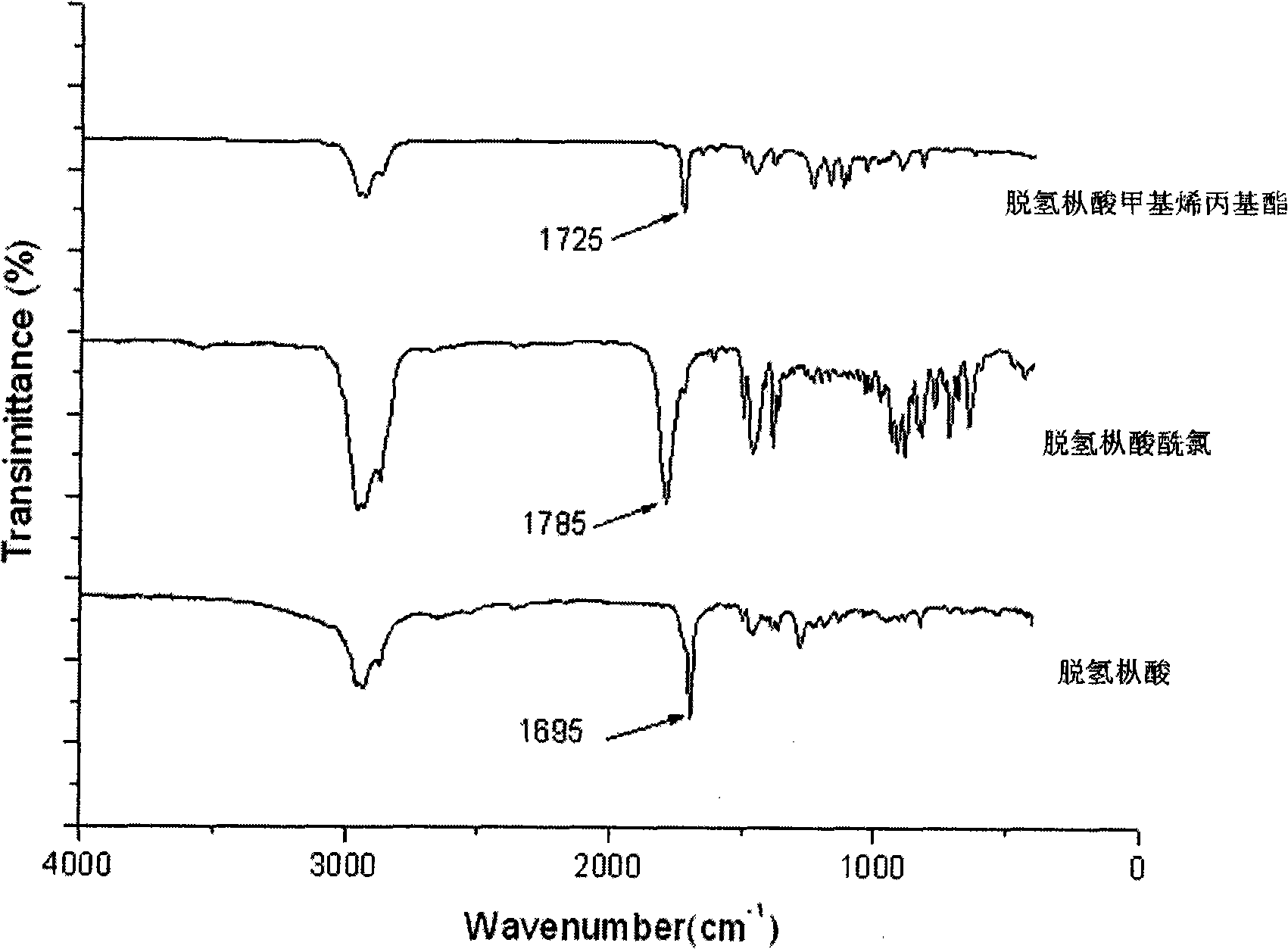
[0029] The first step, the acyl chloride reaction of rosin or rosin derivatives: dissolve one of rosin or rosin derivatives in an aprotic solvent, and the aprotic solvent used is one of benzene, methylene chloride, toluene , and the water content of the solvent is lower than 0.02%, add an acid chloride reagent, heat to make the reaction temperature be 20-85°C, for example, the temperature can be selected as: 5°C, 10°C, 20°C, 30°C, 40°C, 50°C, 52°C, 55°C, 60°C, 70°C, 80°C, react for 0.5-5 hours, the time can be selected as: 0.5 hours, 1 hour, 1.5 hours, 2 hours, 2.5 hours, 3 hours, 3.5 hours, 4 hours, 4.5 hours Hour, 4.8 hours, make the carboxyl acid chloride in rosin or rosin derivative (also can judge according to in infrared spectrum, carboxyl peak disappears, the generation of acid chloride peak, such as for acid chloride reagent, with 1695cm -1 The ...
Embodiment 2
[0032] Example 2 Synthesis of dehydroabietic acid methylallyl ester.
[0033] Methyl allyl dehydroabietate comprises the following steps:
[0034] The first step is the acyl chloride reaction of dehydroabietic acid. Add 10g of dehydroabietic acid (relative molecular weight, 300g / mol) into the reactor, dissolve it with 20g of methylene chloride, and add 4.5g of oxalyl chloride (COCl) at the same time 2 , reacted at 25°C for 3 hours.
[0035] The second step is the esterification reaction of methallyl alcohol. The acid-binding agent pyridine (C 5 h 5 N) 3.42g, polymerization inhibitor p-hydroxyanisole 0.003g and methallyl alcohol 2.4g were added to the reaction product of the first step, and reacted at 50°C for 2-5 hours. The precipitate was filtered off, and the obtained filtrate was vacuum extracted to remove the solvent to obtain methylallyl dehydroabietic acid.
[0036] From the infrared tracking detection of the preparation process of disproportionated rosin methallyl...
Embodiment 3
[0038] Dehydroabietic acid is the main component of disproportionated rosin. After a series of related treatments, dehydroabietic acid can be separated from disproportionated rosin.
[0039] The synthesis of dehydroabietic acid methyl allyl ester comprises the following steps: the first step, the acid chloride reaction of dehydroabietic acid. 10g dehydroabietic acid (molecular weight, 300g / mol) is added in the reactor, dissolves with 20g benzene, adds 5.16g sulfur oxychloride (SOCl 2 ), reacted at 40°C for 3 hours.
[0040] The second step is the esterification reaction of methallyl alcohol. The acid-binding agent sodium carbonate (Na 2 CO 3 ) 5.42g, polymerization inhibitor p-hydroxyanisole 0.003g and methallyl alcohol 2.4g were added to the reaction product of the first step, and reacted at 50°C for 2-5 hours. The precipitate was filtered off, and the obtained filtrate was vacuum extracted to remove the solvent to obtain methylallyl dehydroabietic acid.
[0041]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com