Method for producing hydrocyanic acid (HCN)
A hydrogen cyanide and oxygen technology, which is applied in the field of hydrogen cyanide (HCN) preparation, can solve problems such as damage to catalyst nets, danger of temperature peaks, catalyst destruction, etc., so as to ensure safe operation mode, increase production capacity, and improve yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
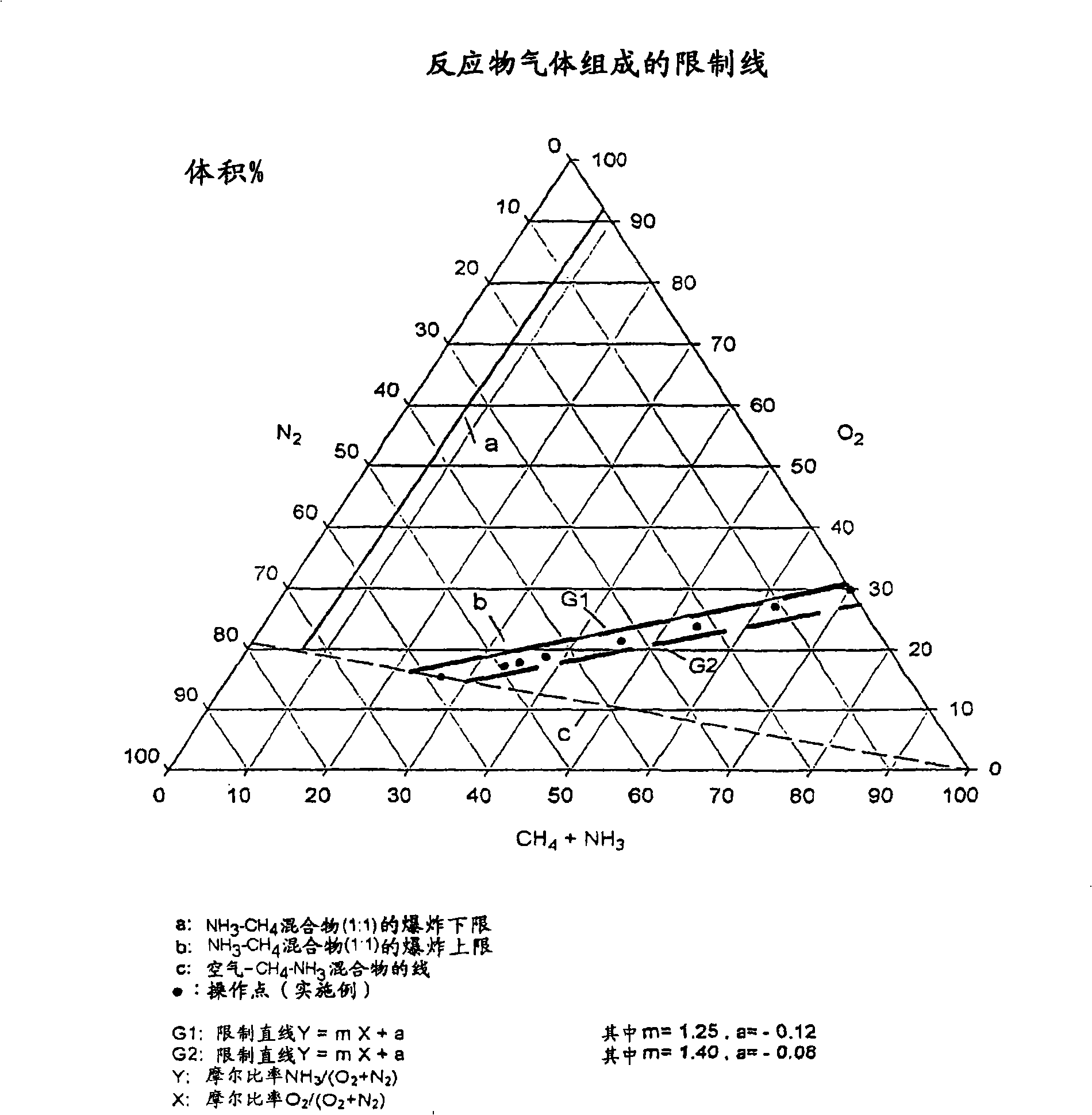
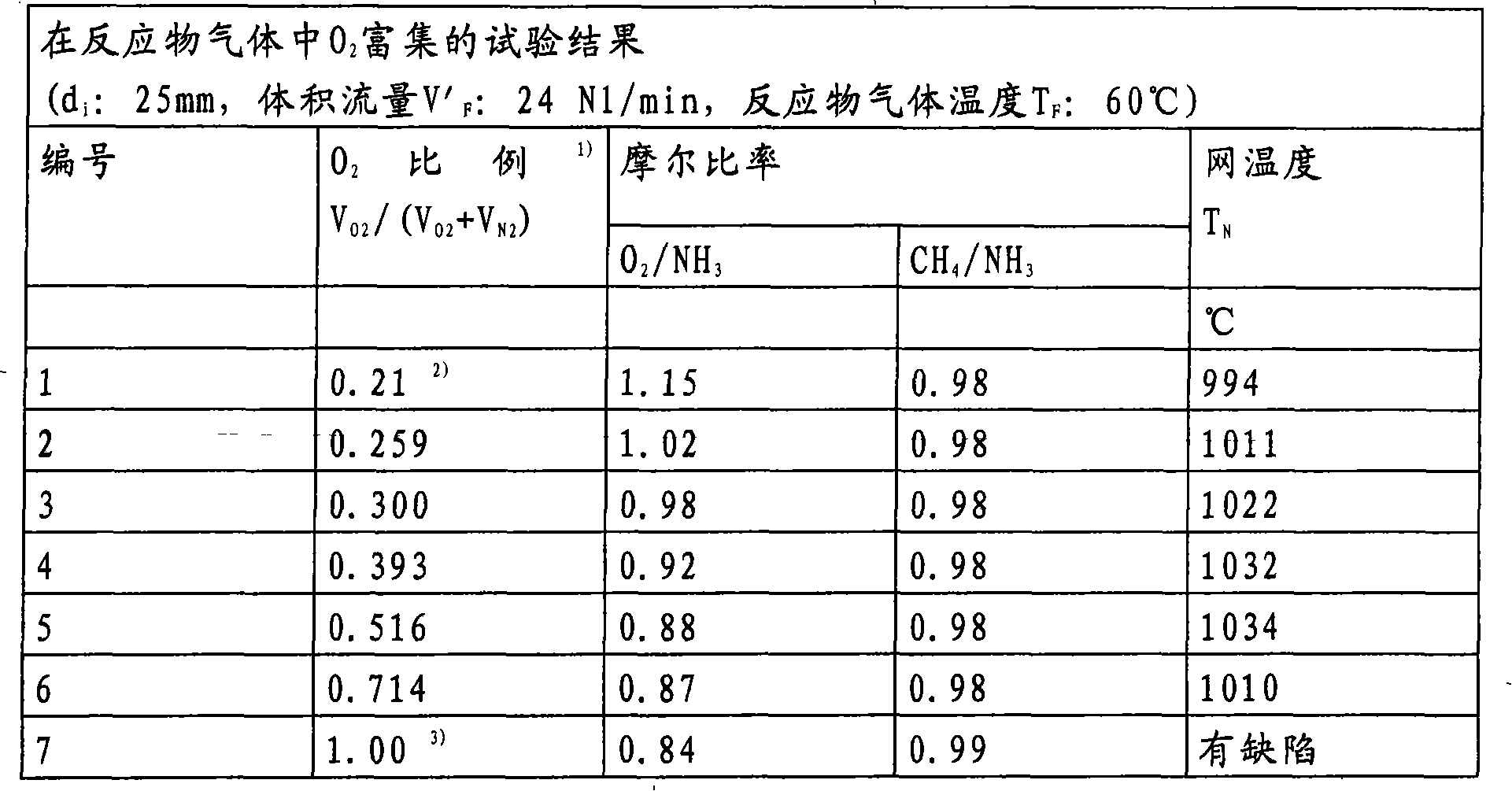
[0062] The following examples were carried out in laboratory equipment consisting of a gas metering system with thermal mass flow regulators for the reactant gases used (methane, ammonia, air, oxygen), for the reaction Electric heater for preheating of the feed gas, reactor part with 6 layers of Pt / Rh10 catalyst mesh (inner diameter d: 25 mm) and downstream HCN scrubber for neutralization of formed HCN with NaOH solution.
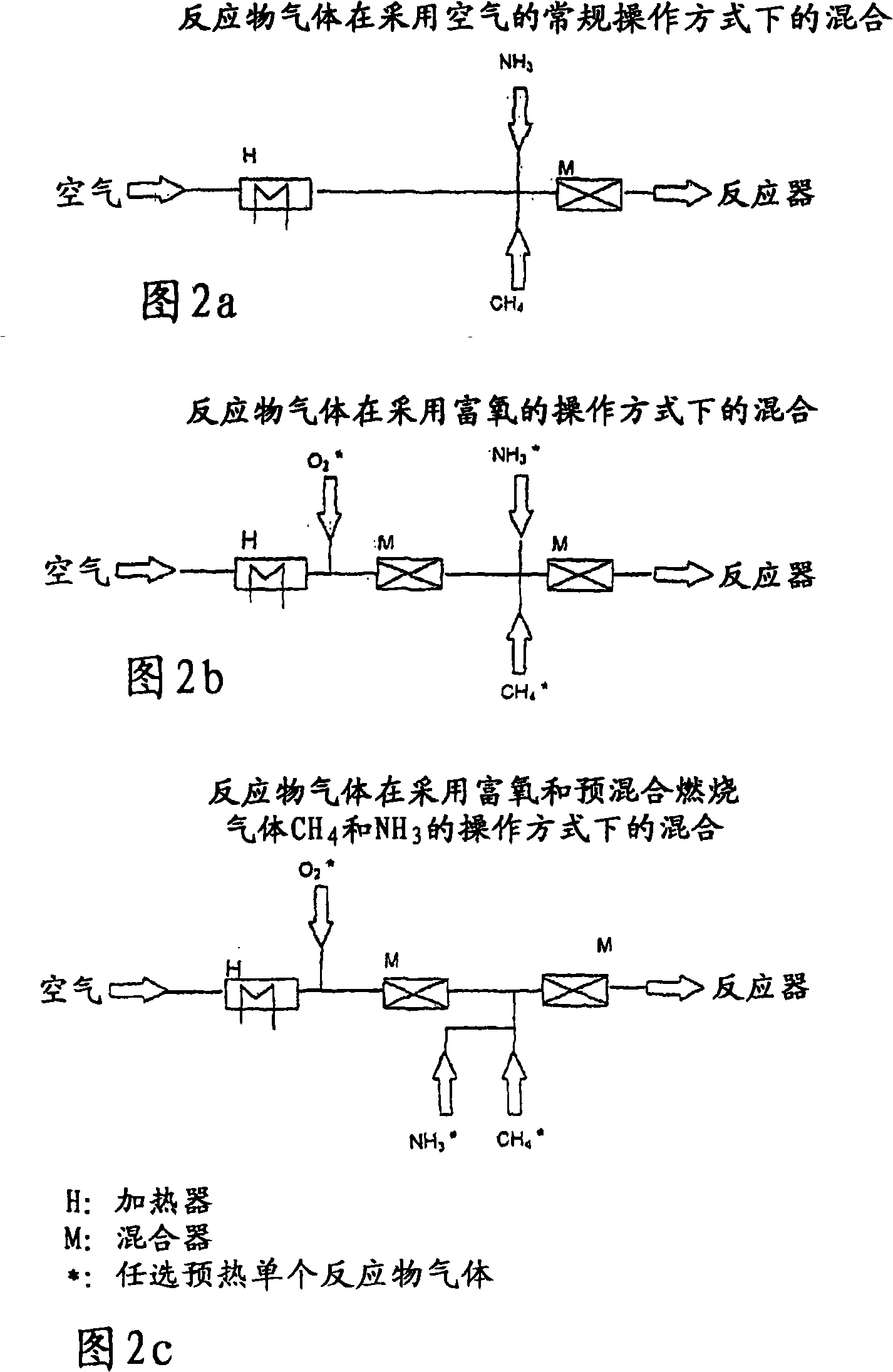
[0063] The reaction gases were analyzed online by GC. In order to account for the amount of HCN formed, the CN content in the effluent of the HCN scrubber was also determined by argentometric titration. Starting from a mode of operation corresponding to known operating conditions using air as the source of oxygen, air oxygen was gradually replaced by pure oxygen in the test series, while at constant CH 4 / NH 3 Molar O 2 / NH 3 The ratio is reduced. All experiments were performed with a constant reactant gas volume flow of 24 N1 (standard liter) / min.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com