Six-membered ring fragrant trianhydride and preparation method thereof
A six-membered ring and aromatic technology, which is applied in the field of six-membered ring aromatic trianhydrides and its preparation, has achieved wide application prospects and excellent mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
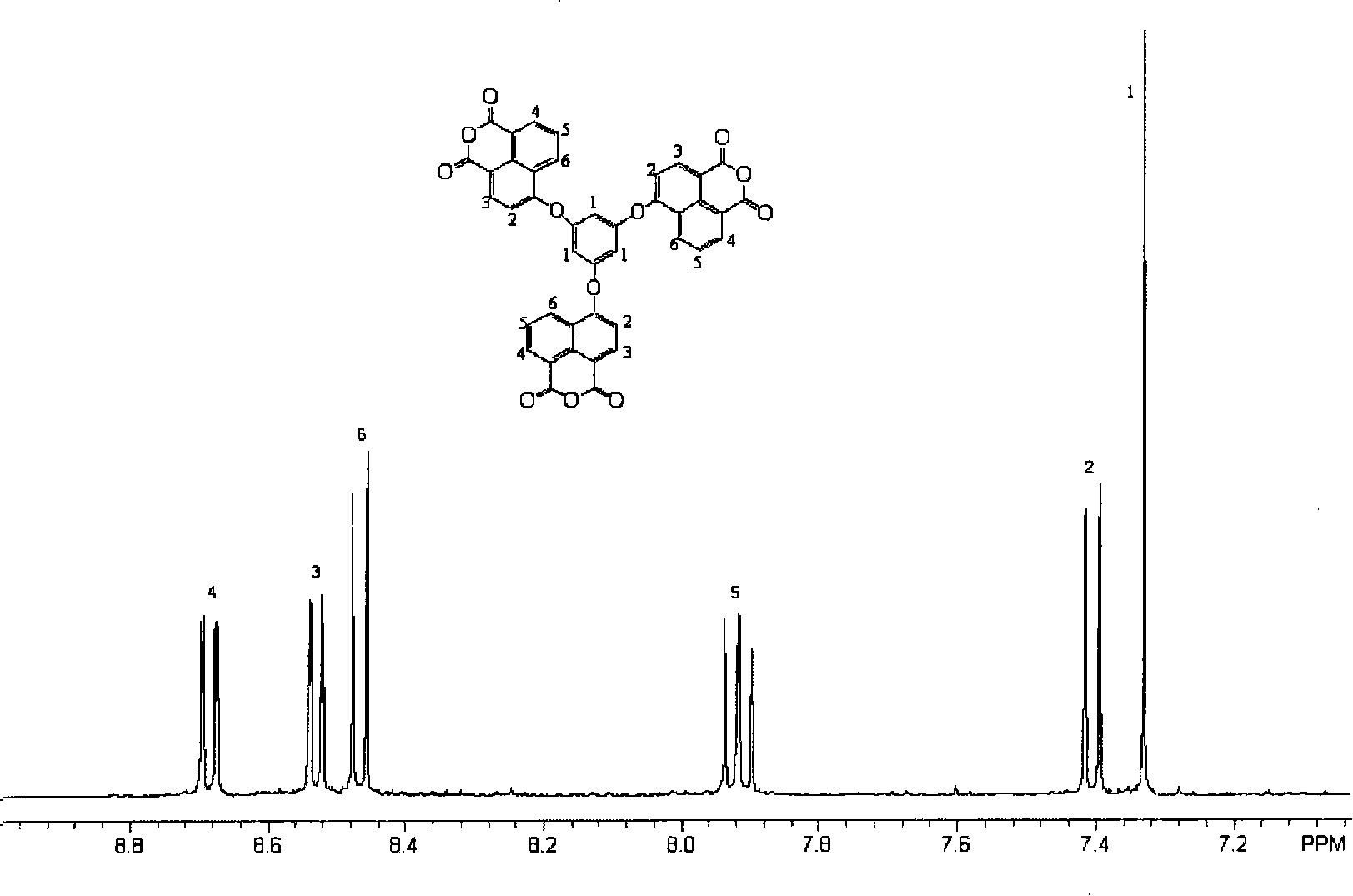
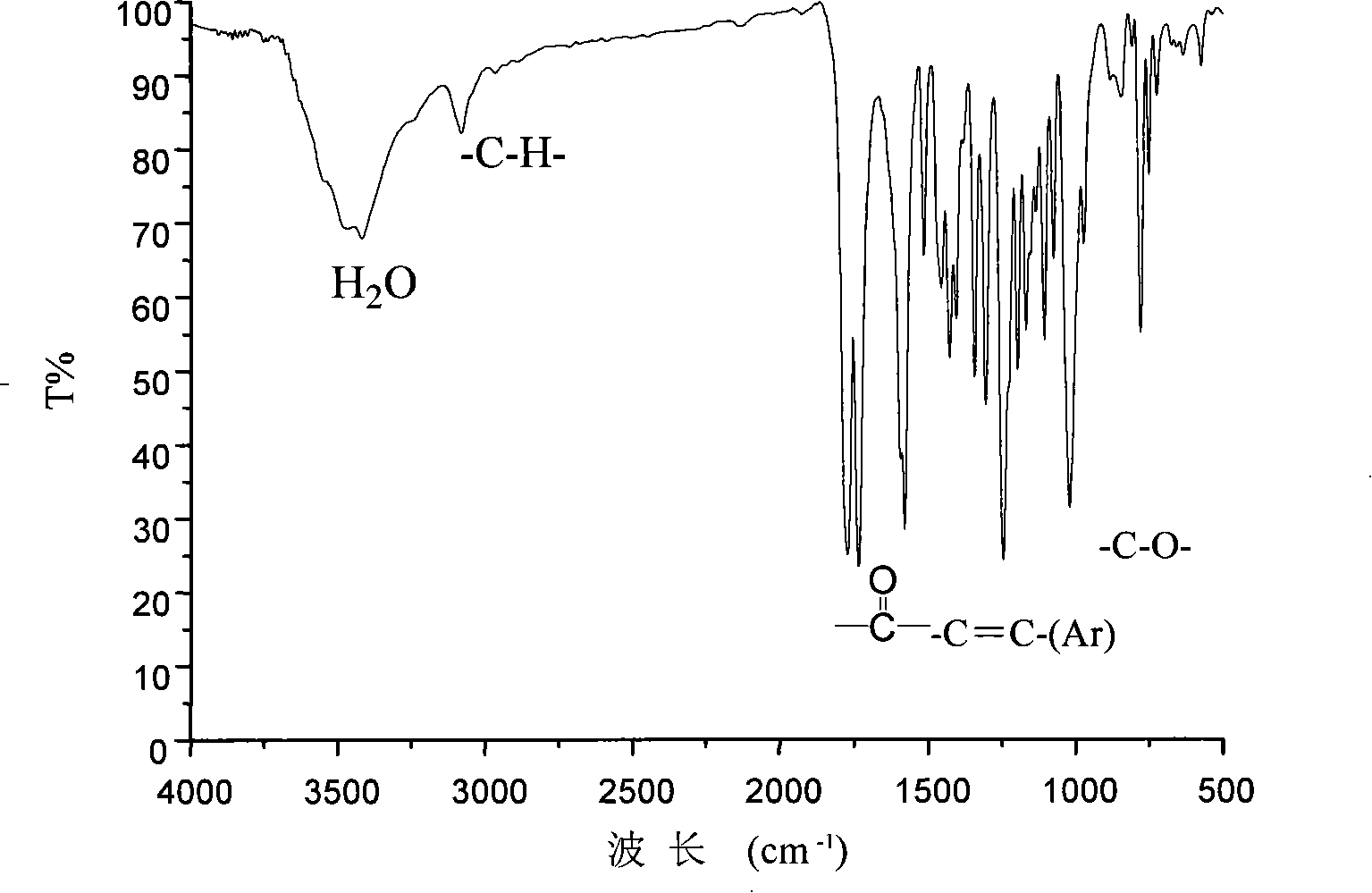
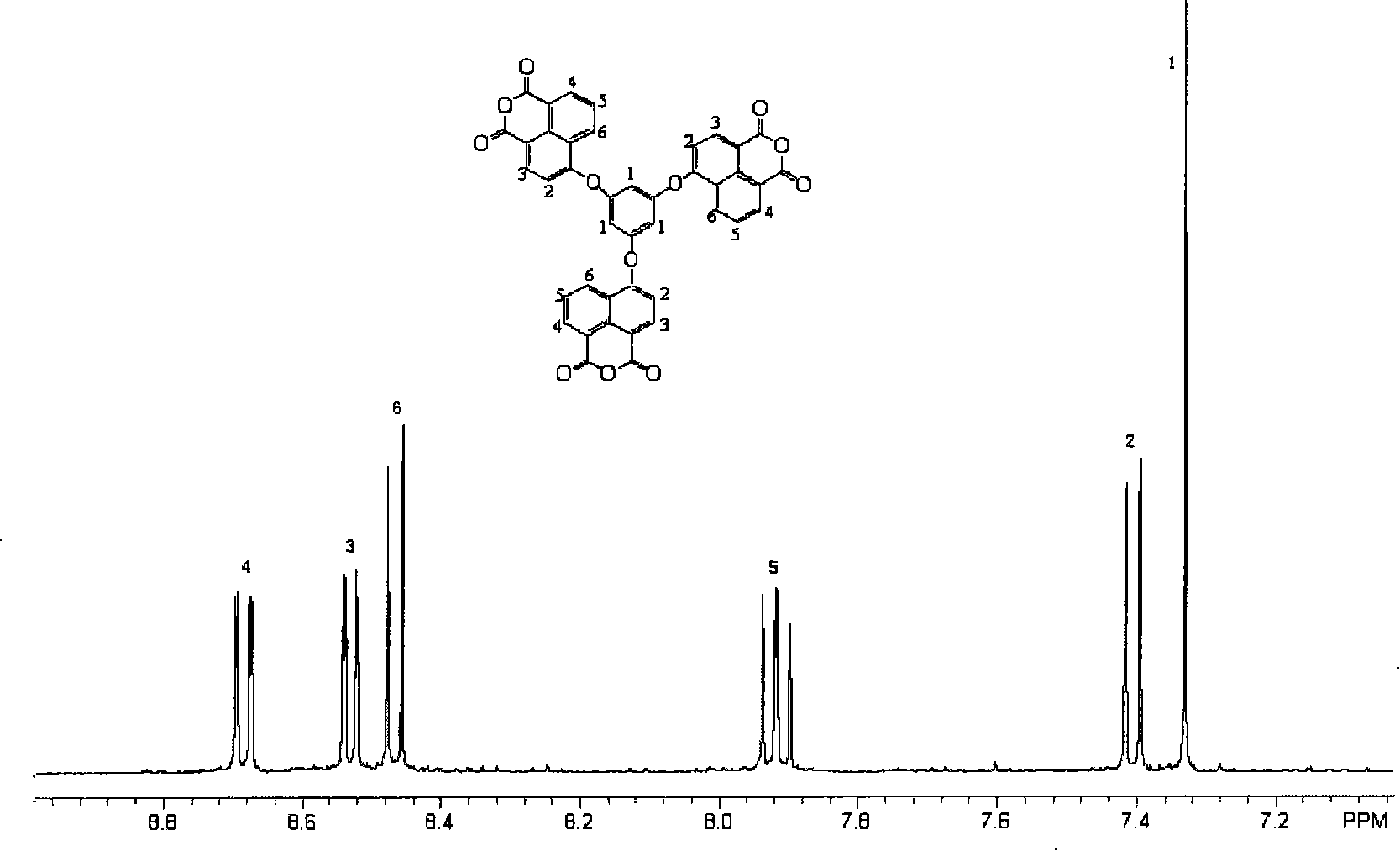
[0022] In a 100mL three-necked flask equipped with nitrogen inlet, magnetic stirring, water separator and reflux condenser, add 0.6305g (5mmol) 1,3,5-trihydroxybenzene, 15mL methanol, 0.60g (15mmol) sodium hydroxide , heated up to 100°C, evaporated methanol and water, evaporated to dryness, cooled to room temperature, added 4.156 grams (15 mmol) of 4-bromo-1,8-naphthalene anhydride, 16 mL of N-methylacetamide to the reaction flask, and heated To 120°C, drop 15mL of toluene into the reaction system through the dropping funnel, raise the temperature to 140°C, react for 4 hours, distill off the toluene, raise the temperature to 150°C, and continue the reaction for 18 hours. After the reaction, the system is cooled to room temperature. Then the resultant was poured into 150 mL of acetone, and the precipitated solid in the system was washed with water, filtered, and vacuum-dried to obtain 2.87 g of crude product. The obtained crude product was recrystallized with acetic anhydride t...
Embodiment 2
[0027] In a 100mL three-necked flask equipped with nitrogen inlet, magnetic stirring, water separator and reflux condenser, add 0.3783g (3mmol) 1,3,5-trihydroxybenzene, 9mL methanol, 0.36g (9mmol) sodium hydroxide , heated up to 100°C, evaporated methanol and water, evaporated to dryness, cooled to room temperature, added 2.494 g (9 mmol) 4-bromo-1,8-naphthalene anhydride, 6.35 mL N-methylacetamide to the reaction flask, Raise the temperature to 120°C, drop 10mL of toluene into the reaction system through the dropping funnel, raise the temperature to 140°C, react for 4 hours, distill off the toluene, raise the temperature to 150°C, and continue the reaction for 20 hours. After the reaction, the system was cooled to room temperature. Then the resultant was poured into 100 mL of acetone, and the solid precipitated in the system was washed with water, filtered, and vacuum-dried to obtain 1.79 g of a crude product. The obtained crude product was recrystallized with acetic anhydrid...
Embodiment 3
[0029] In a 100mL three-necked flask equipped with nitrogen inlet, magnetic stirring, water separator and reflux condenser, add 0.6305g (5mmol) 1,3,5-trihydroxybenzene, 8mL methanol, 0.60g (15mmol) sodium hydroxide , heated up to 100°C, evaporated methanol and water, evaporated to dryness, cooled to room temperature, added 4.156 grams (15 mmol) of 4-bromo-1,8-naphthalene anhydride, 10 mL of N-methylacetamide to the reaction flask, and heated To 120°C, drop 15mL of toluene into the reaction system through the dropping funnel, raise the temperature to 140°C, react for 4 hours, distill off the toluene, raise the temperature to 150°C, and continue the reaction for 22 hours. After the reaction, the system is cooled to room temperature. Then the resultant was poured into 100 mL of acetone, and the precipitated solid in the system was washed with water, filtered, and vacuum-dried to obtain 2.58 g of crude product. The obtained crude product was recrystallized with acetic anhydride to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com