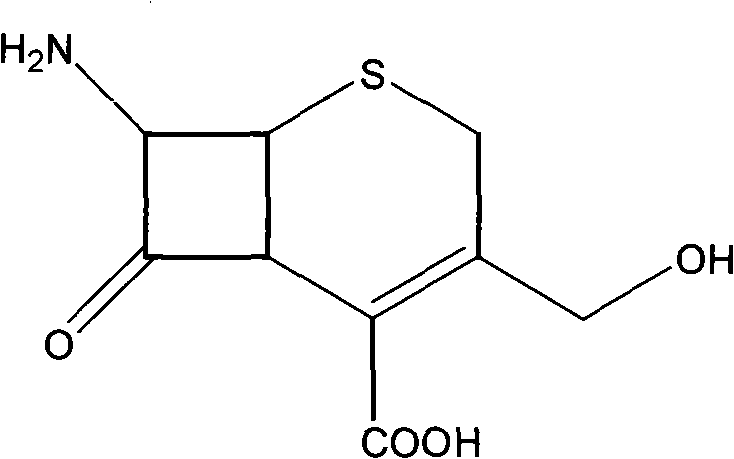
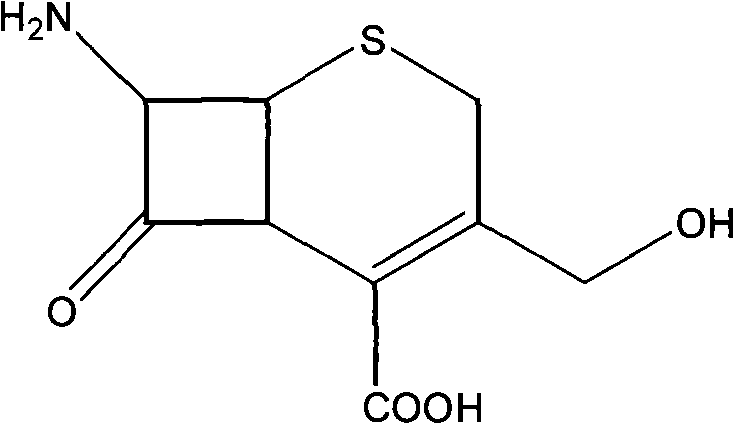
7-amido-3-hydroxyl methyl-3-cephalosporin-4-carboxylic acid crystal and preparing method thereof
A hydroxymethyl, cephalosporin technology, applied in organic chemistry and other directions, to achieve the effects of good stability, easy transportation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Add 7-ACA to the mixture of water and methanol below 0°C, slowly add 1N NaOH, adjust the pH to 7-9, dissolve the solution, and maintain the reaction for 10-20 hours. After the completion of the reaction, slowly add 6N HCl to adjust the pH to 4 to 4.5 crystallization. Stir and grow the crystal for 1 hour, filter with suction, wash twice with water, wash with acetone slurry, and then dry at 40°C to obtain the crude product.
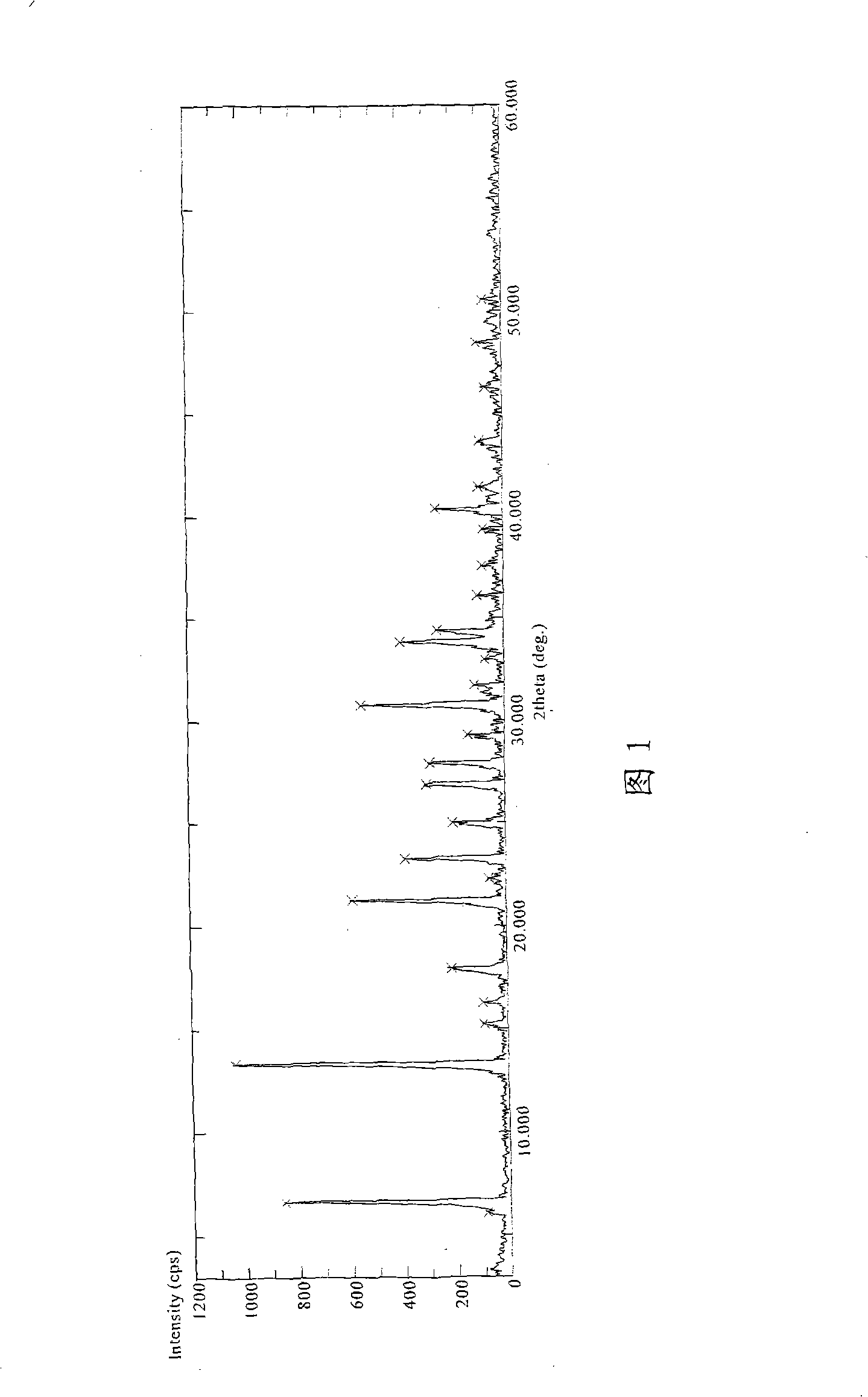
[0018] Put 10 grams of the above crude product and 200 milliliters of water into the reaction bottle, cool down to less than 5°C in an ice-water bath, add 10% NaOH solution dropwise until the solution is clear, pH=9, add 2 grams of activated carbon, stir for 30 minutes to decolorize, filter, stir the filtrate, Slowly add 3N hydrochloric acid dropwise at 25°C to make the pH of the feed liquid 3.5, filter, wash twice with water, wash with acetone slurry, and vacuum dry at 35°C to obtain 8.16 g of the product of the present invention. HPLC d...
Embodiment 2
[0022] Example 2: 10 grams of the crude product obtained by the above method, 100 milliliters of methanol and 100 milliliters of water were put into a reaction flask, the temperature was lowered to -10° C., ammonia water was added dropwise until the solution was clear, pH=7.5, 2 grams of active carbon was added, and stirred for 30 minutes to decolorize. Filter carbon, then stir the filtrate, slowly add 3N hydrochloric acid dropwise at 0°C to make the pH of the feed solution 4.0, crystallize, filter, wash with water twice, and dry in vacuum at 35°C to obtain 7.38 g of the product. X-ray powder diffraction data are the same as in Example 1.
Embodiment 3
[0023] Example 3: 10 grams of the crude product obtained by the above method, 50 milliliters of acetonitrile and 100 milliliters of water were put into a reaction flask, protected by nitrogen gas, 12% ethylenediamine solution was added dropwise until dissolved, 2 grams of activated carbon was added, stirred for 30 minutes for decolorization, and filtered , stirred the filtrate, slowly added dilute sulfuric acid dropwise at 10°C to make the pH of the feed solution = 4.5, filtered, washed twice with water, and dried in vacuum at 40°C to obtain 8.10 g of the product. X-ray powder diffraction data are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com