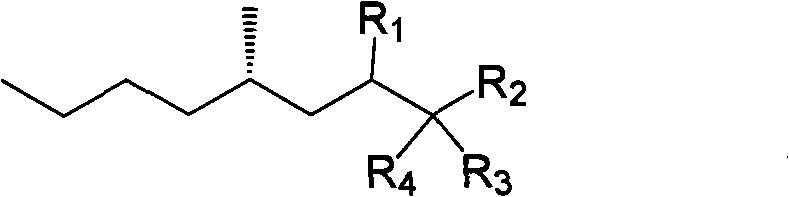
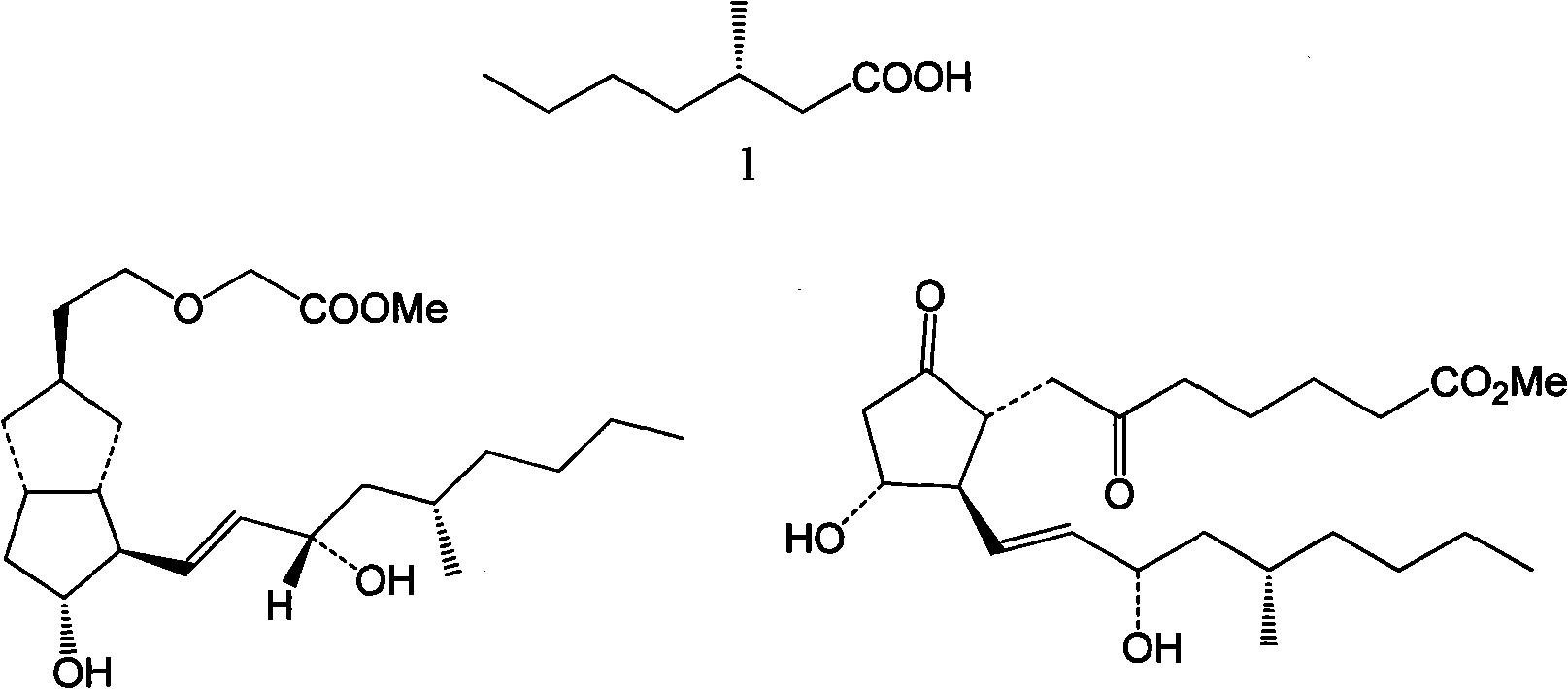
S-4- methyloctane derivate, synthetic method thereof and application thereof to (S)-3-methyl-heptanoate sythesis
A technology of methyl octane and synthesis method, applied in the directions of organic chemistry methods, chemical instruments and methods, preparation of organic compounds, etc., can solve problems such as complicated methods, and achieve the effects of simple operation and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
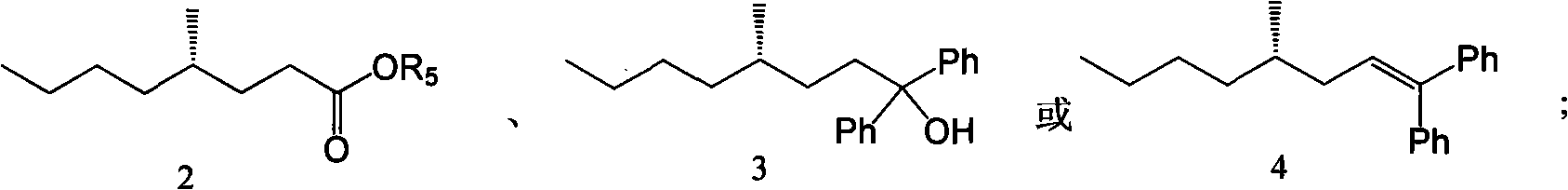
[0028] The synthesis of embodiment 1 compound 2a
[0029]
[0030] Take a 50mL bottle, weigh metal magnesium (0.632g, 26mmol) in it, add 20mL of freshly distilled tetrahydrofuran under the protection of argon, and then add dropwise a solution containing n-propyl bromide (2.2mL, 24mmol, 2.4eq.) under reflux. 20mL of freshly steamed tetrahydrofuran solution, after the dropwise addition, continue to stir for 1h;
[0031] Take a 250mL three-neck flask, vacuumize and fill it with argon three times, add anhydrous tetrahydrofuran 25ml, compound 5a (2.03g, 10mmol, 1eq.), N-methyl-2-pyrrolidone ( NMP) (3.6ml, 20.9mmol, 4eq.), and Li 2 CuCl 4 solution in THF (0.6 mL, 1.5 mol / L, 1 mmol). Add the Grignard reagent of n-propylmagnesium bromide into the constant-pressure dropping funnel, slowly drop it into the system at room temperature, and react at room temperature for about 4 hours after the addition, add saturated ammonium chloride solution to the reaction solut...
Embodiment 2
[0032] The synthesis of embodiment 2 compound 2b
[0033]
[0034]Take a 50mL bottle, weigh metal magnesium (0.632g, 26mmol) in it, add 20mL of freshly distilled tetrahydrofuran under the protection of argon, and then add dropwise a solution containing n-propyl bromide (2.2mL, 24mmol, 2.4eq.) under reflux. 20mL of freshly steamed tetrahydrofuran solution, after the dropwise addition, continue to stir for 1h;
[0035] Take a 250mL three-neck flask, vacuumize and fill it with argon three times, add anhydrous tetrahydrofuran 25ml, compound 5b (2.71g, 10mmol, 1eq.), N-methyl-2-pyrrolidone ( NMP) (3.6ml, 20.9mmol, 4eq.), and Li 2 CuCl 4 solution in THF (1 mL, 1.5 mol / L, 1.5 mmol). Add the Grignard reagent of n-propylmagnesium bromide into the constant pressure dropping funnel, slowly drop it into the system at room temperature, after the dropwise addition, react at room temperature for about 7 hours, add saturated ammonium chloride solution to the reaction so...
Embodiment 3
[0036] The synthesis of embodiment 3 compound 3
[0037]
[0038] Take a 100mL bottle, weigh metal magnesium (0.322g, 13.4mmol) into it, add 15mL of freshly distilled tetrahydrofuran under the protection of argon, and then add dropwise 5mL of freshly distilled tetrahydrofuran containing bromobenzene (1.4mL, 13.3mmol, 2.3eq.) The tetrahydrofuran solution, continue to stir for 0.5h after the dropwise addition. Add compound 2a (0.99g, 5.76mmol, 1eq.) 10mL freshly steamed tetrahydrofuran solution dropwise, react at room temperature for 6h, add 1.2M hydrochloric acid to quench the reaction, and then separate the liquids, extract the aqueous phase with ethyl acetate three times, combine the organic phases, Wash once with saturated sodium bicarbonate, once with saturated brine, dry over anhydrous sodium sulfate, filter, spin off the solvent under reduced pressure, and obtain 1.426 g of compound 3 with a yield of 84% by silica gel column chromatography. [α] D ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com