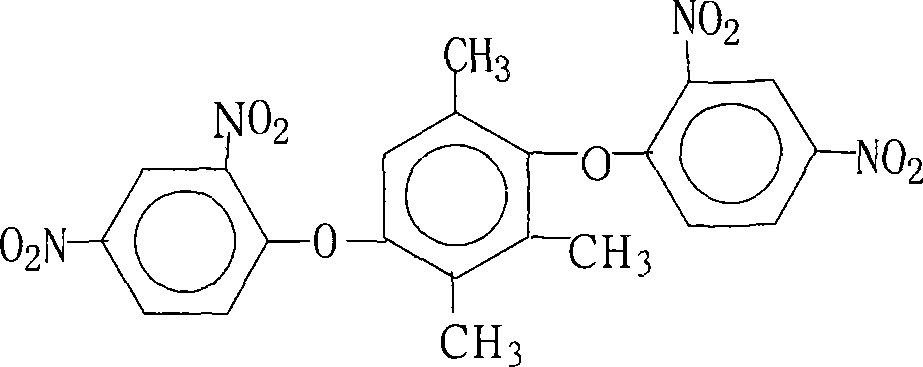
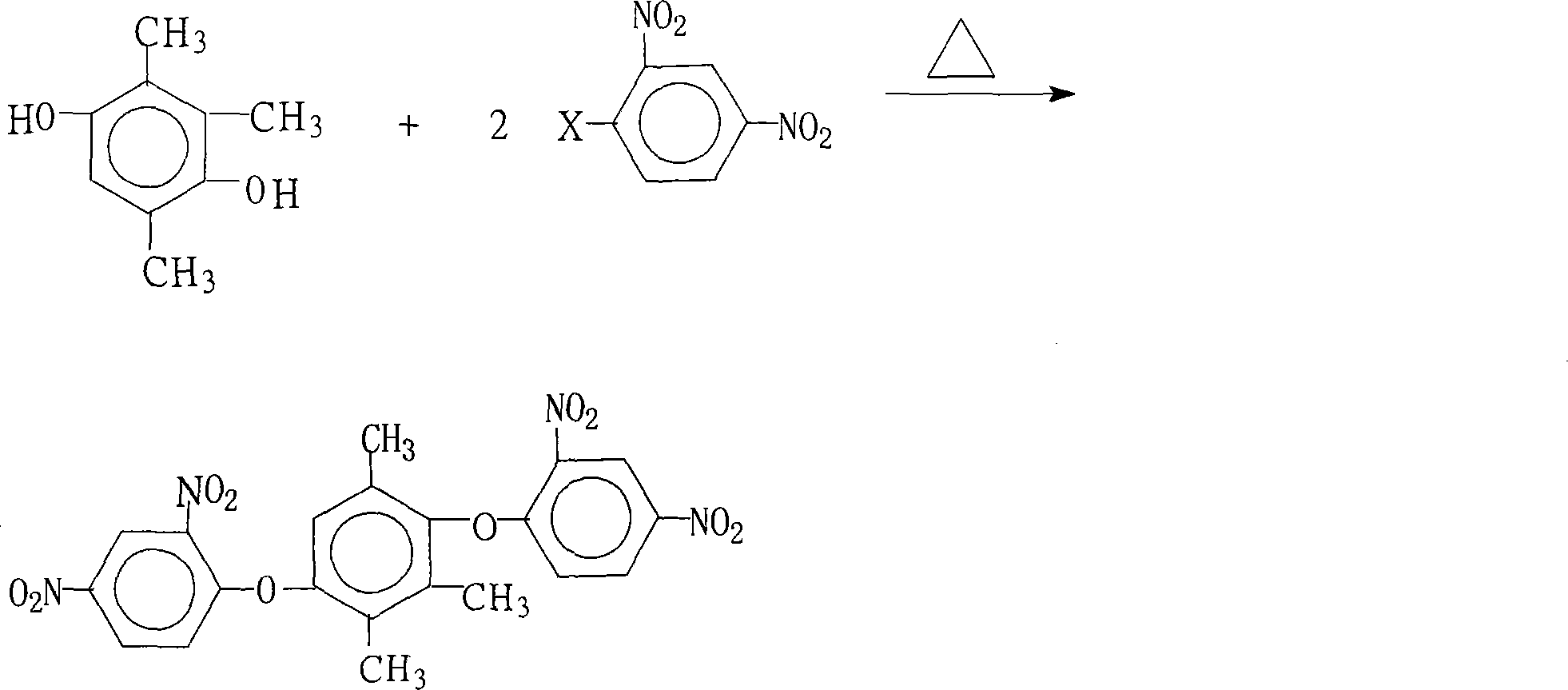
Preparation of 1,4-bis(2,4- dinitrophenoxy)-2,3,5- trimethylbenzene
A technology of dinitrophenoxy and trimethylbenzene, applied in 1 field, achieves the effects of high yield and purity, few types of use, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 15.2 grams (0.10 moles) of 2,3,5-trimethylhydroquinone, 44.6 grams (0.22 moles) of 2,4-dinitrochlorobenzene, 110.4 grams (0.80 moles) of potassium carbonate, 1216 milliliters of N , N-dimethylformamide and 130 milliliters of benzene were put into the reaction kettle, stirred, heated to reflux and separated from water for 18 hours, concentrated the reaction solution, recovered the solvent for recycling, cooled the reactant system, added water, and precipitated a solid product. Wash 2 to 3 times with hot water, dry to obtain 45.1 grams of 1,4-bis(2,4-dinitrophenoxy)-2,3,5-trimethylbenzene with a purity of 99.4%. The amount and theoretical amount (48.4 g) of 1,4-bis(2,4-dinitrophenoxy)-2,3,5-trimethylbenzene obtained was calculated to give 1,4-bis(2,4 The yield of -dinitrophenoxy)-2,3,5-trimethylbenzene was 93.2%.
Embodiment 2
[0028] 15.2 grams (0.10 moles) of 2,3,5-trimethylhydroquinone, 54.4 grams (0.22 moles) of 2,4-dinitrobromobenzene, 55.2 grams (0.40 moles) of potassium carbonate, 10.6 grams ( 0.10 mole) of sodium carbonate, 600 milliliters of N, N-dimethylacetamide, 160 milliliters of benzene and 30 milliliters of dimethylbenzene were put into the reaction kettle, stirred, and after heating and reflux water separation reaction for 6 hours, the reaction solution was concentrated, and the solvent was recovered to Recycle, cool the reactant system, add water, precipitate a solid product, wash with hot water 2 to 3 times, and dry to obtain 37.8 grams of 1,4-bis(2,4-dinitrophenoxy)-2,3, 5-trimethylbenzene, the purity is 99.5%, according to the actual amount and theoretical amount (48.4 grams), the calculated yield of 1,4-bis(2,4-dinitrophenoxy)-2,3,5-trimethylbenzene was 78.2%.
Embodiment 3
[0030] 15.2 grams (0.10 moles) of 2,3,5-trimethylhydroquinone, 40.5 grams (0.20 moles) of 2,4-dinitrochlorobenzene, 10.6 grams (0.10 moles) of sodium carbonate, 76 milliliters of N -Methyl-2-pyrrolidone, 100 milliliters of benzene and 52 milliliters of dichlorobenzene were put into the reaction kettle, stirred, heated to reflux and separated from water for 12 hours, concentrated the reaction solution, recovered the solvent for recycling, cooled the reactant system, Add water, separate out solid product, wash 2~3 times with hot water, dry, obtain 36.6 grams of 1,4-bis(2,4-dinitrophenoxy)-2,3,5-trimethylbenzene, purity Be 99.7%, according to the amount of 1,4-bis(2,4-dinitrophenoxy)-2,3,5-trimethylbenzene actually obtained and theoretical yield (48.4 grams), calculate 1, The yield of 4-bis(2,4-dinitrophenoxy)-2,3,5-trimethylbenzene was 75.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com