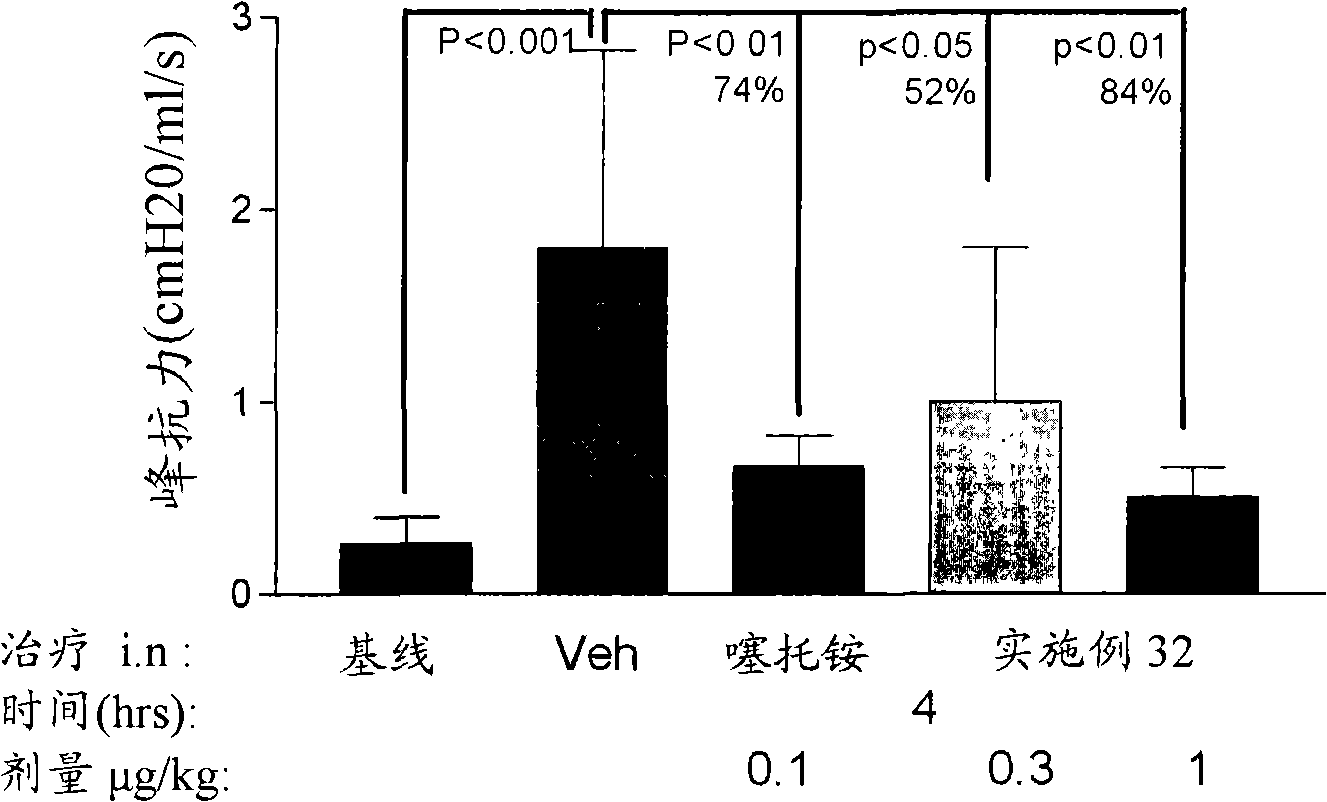
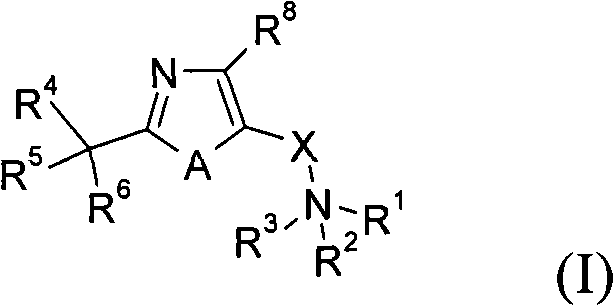
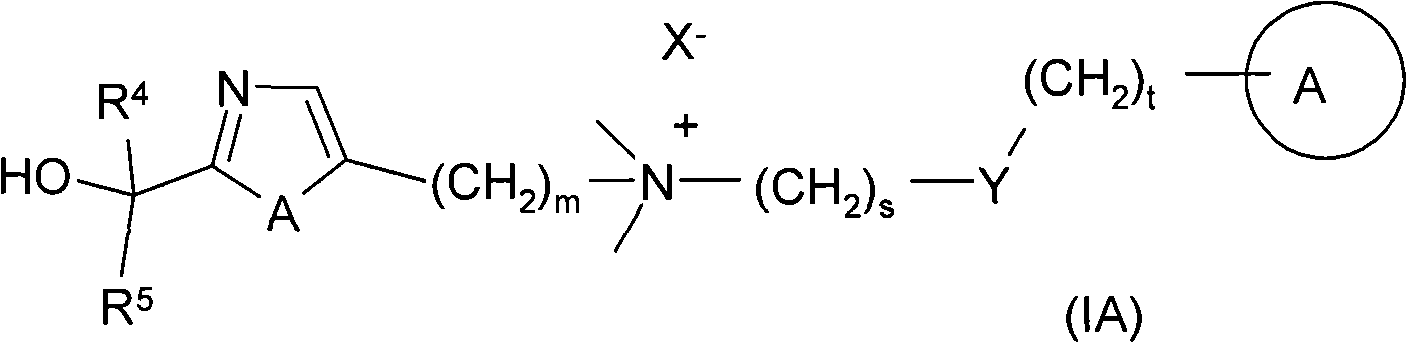Azole and thiazole derivatives and their use
一种化合物、药用盐的技术,应用在噁唑和噻唑衍生物领域,能够解决很少抗毒蕈碱化合物等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0619]
[0620] (5-Dimethylaminomethyl-oxazol-2-yl)-diphenyl-methanol (I-a): R a , R b = Ph, R c , R d =CH 3
[0621] Phenylmagnesium bromide (0.75ml in 1M THF, 0.75mmol) was added dropwise to (5-dimethylaminomethyl-oxazol-2-yl)-phenyl-methanone (Intermediate 4) ( 0.15 g, 0.65 mmol) in cold (0° C.) solution in anhydrous THF (1.5 ml). The mixture was stirred and cooled for 1.5 hours, then additional phenylmagnesium bromide (0.4ml of a 1M solution in THF, 0.4mmol) was added dropwise. The mixture was stirred at 0°C for 0.5 h, then treated with excess saturated aqueous ammonium chloride solution. The mixture was extracted with DCM (x2), the combined organic phases were washed with brine and dried (Na 2 SO 4 ) and removal of the solvent gave the crude product. Purified by HPLC using 5-70% acetonitrile / water with 0.1% TFA over 18.5 minutes.
[0622] Yield: 0.19g (69%, as its TFA salt)
[0623] LC-MS (Method 1): Rt 5.56 min, m / z 309 [MH + ].
[0624] LC-MS (Method 3):...
Embodiment 2
[0630]
[0631] [2-(Hydroxy-diphenyl-methyl)-oxazol-5-ylmethyl]-dimethyl-(3-phenoxy-propyl)-ammonium formate (I-b): R a = R b = Ph, R c , R d =CH 3 , R e = 3-phenoxypropyl
[0632] A solution of (5-dimethylaminomethyl-oxazol-2-yl)-diphenyl-methanol (Example 1) (24 mg, 0.078 mmol) in acetonitrile (0.3 mL) and chloroform (0.5 mL) Treated with 3-phenoxybromopropane (37 μL, 0.23 mmol), the reaction mixture was stirred at room temperature overnight and then at 50° C. for 42 hours. The volatiles were removed by evaporation and the crude product was purified by preparative HPLC using 25-75% acetonitrile / water containing 0.1% formic acid over 30 minutes to give the product as a colorless gum.
[0633] Yield 24mg, 63%
[0634] LC-MS (Method 1): Rt 7.56 min m / z 443 [M + ]
[0635] 1 H NMR (MeOD): δ2.29(m, 2H), 3.11(s, 6H), 3.45(m, 2H), 3.98(t, 2H), 4.79(s, 2H), 6.85-6.90(m, 2H ), 6.93-6.98 (m, 1H), 7.24-7.38 (m, 12H), 7.56 (s, 1H), 8.51 (br s, 1H).
[0636] The following ...
Embodiment 4
[0642]
[0643] Cyclohexyl-(5-dimethylaminomethyl-oxazol-2-yl)-phenyl-methanol (I-a): R a ,=Ph,R b = cyclohexyl, R c , R d =CH 3
[0644] A solution of (5-bromomethyl-oxazol-2-yl)-cyclohexyl-phenyl-methanol (Intermediate 9) (3.2 g, 9.2 mmol) in 40 mL THF was treated with 2M dimethylamine in THF (40 mL, 80 mmol ) solution treatment. A suspension formed after stirring for several minutes. The reaction mixture was allowed to stand overnight at room temperature, then the solid was filtered and discarded. After the filtrate was concentrated under reduced pressure, the residue was partitioned between DCM and saturated sodium bicarbonate solution. The organic layer was dried (Na 2 SO 4 ) to afford the title compound as a solid.
[0645] Yield: 2.74g (95%)
[0646] LC-MS (Method 1): Rt 6.57 min, m / z 315 [MH + ].
[0647] 1 H NMR (DMSO-d 6 ): δ0.92-1.29(m, 6H), 1.42-1.74(m, 4H), 2.10(s, 6H), 2.22(m, 1H), 3.45(s, 2H), 5.90(s, 1H), 6.98 (s, 1H), 7.18-7.22 (m, 1H), 7.27...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com