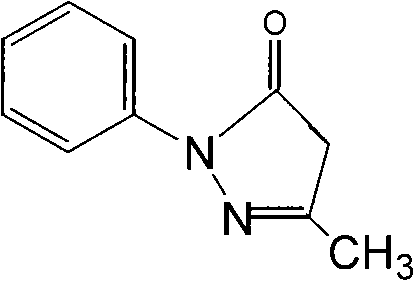
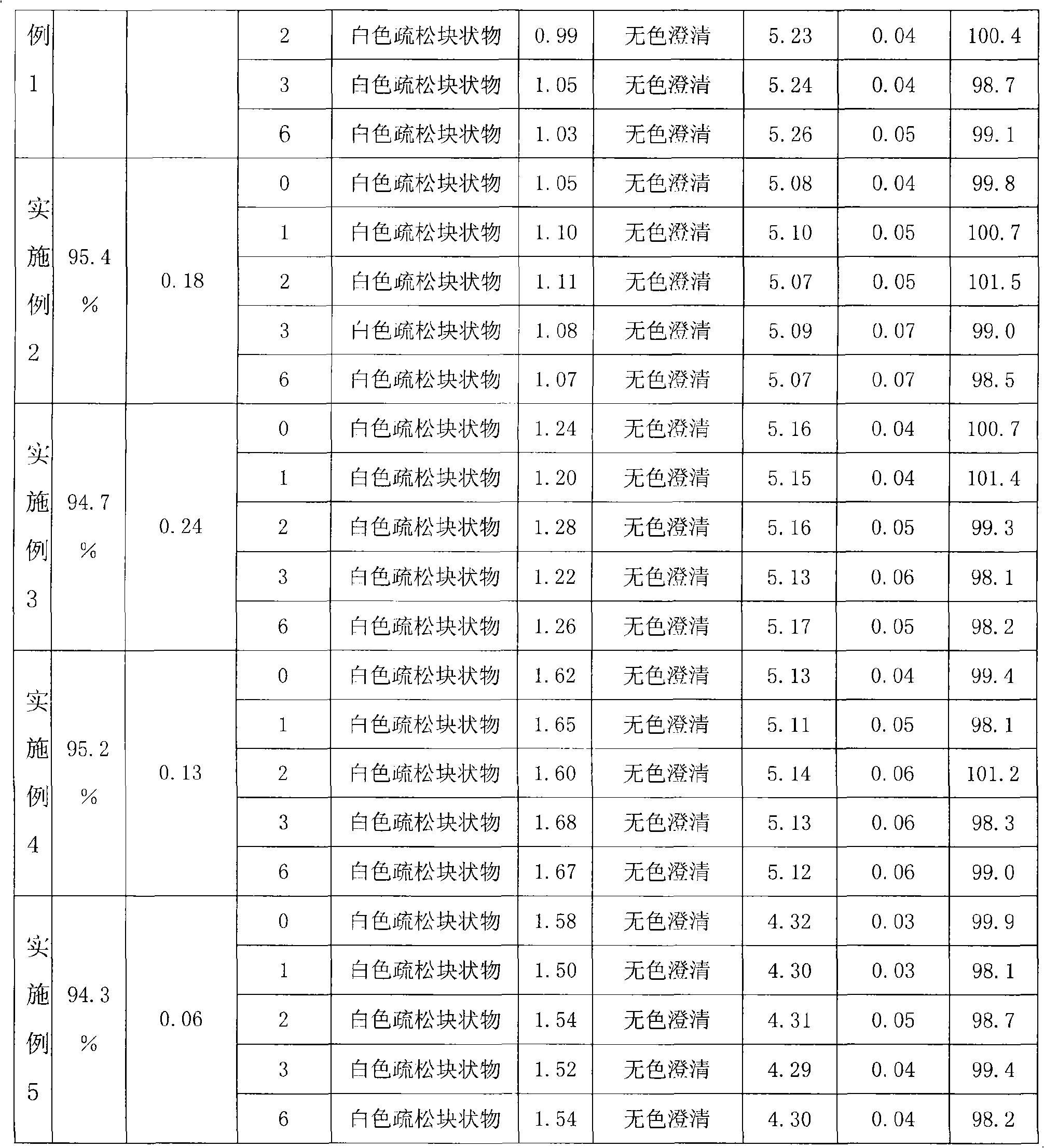Edaravone lyophilized preparation and preparation technique thereof
A freeze-dried preparation and preparation process technology, applied in the field of pharmaceutical preparations, can solve the problems of low drug concentration, difficulty in actual production, difficulty in determining whether the improvement of solubility can meet the requirements of actual use, etc., and achieve the effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
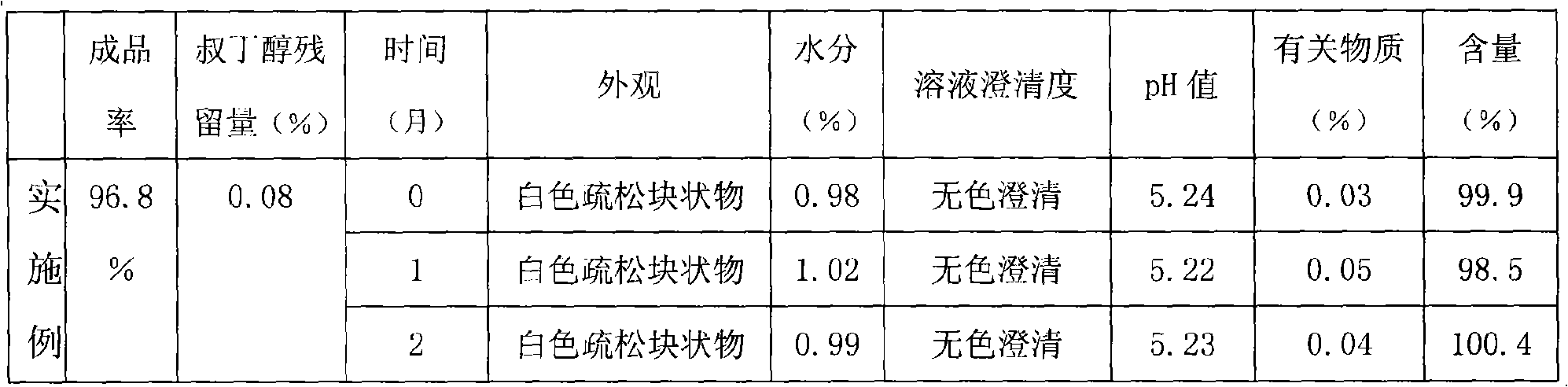
Embodiment 1
[0028] prescription:
[0029] Edaravone 10g
[0030] Mannitol 200g
[0031] tert-butanol 750ml
[0032] Add water for injection to 5000ml
[0033] Preparation: Take the prescribed amount of Edaravone, add 750ml of tert-butanol (if the room temperature is low, tert-butanol is a solid, it can be heated at 35°C to dissolve and then used), stir to dissolve, add water for injection to 5000ml, and stir evenly , add the prescribed amount of mannitol, stir to dissolve, and obtain the drug-containing solution. Add 0.1% activated carbon and stir at 40°C for 30 minutes, filter to decarbonize, then filter through a 0.22um microporous membrane, pack in vials, and freeze-dry according to the freeze-drying process.
Embodiment 2
[0035] prescription:
[0036] Edaravone 75g
[0037] Lactose 125g
[0038] tert-butanol 2250ml
[0039] Add water for injection to 5000ml
[0040] Preparation: Take the prescribed amount of Edaravone, add 2250ml of tert-butanol (if the room temperature is low, tert-butanol is solid, it can be heated at 35°C to dissolve and then used), stir to dissolve. Get the lactose of the prescribed amount, add water for injection to 5000ml, stir to dissolve, mix with tert-butanol solution evenly, and obtain the drug-containing solution. Add 0.1% activated carbon and stir at 40°C for 30 minutes, filter to decarbonize, then filter through a 0.22um microporous membrane, pack in vials, and freeze-dry according to the freeze-drying process.
Embodiment 3
[0042] prescription:
[0043] Edaravone 10g
[0044] Mannitol 200g
[0045] Sucrose 200g
[0046] tert-butanol 1750ml
[0047] Add water for injection to 5000ml
[0048]Preparation: Take the prescribed amount of Edaravone, add 1750ml of tert-butanol (if the room temperature is low, tert-butanol is a solid, it can be heated at 35°C to dissolve and then taken), stir to dissolve, add water for injection to 5000ml, stir evenly , add the prescribed amount of mannitol and sucrose, stir to dissolve, and obtain the drug-containing solution. Add 0.1% activated carbon and stir at 40°C for 30 minutes, filter to remove carbon, then filter through a 0.22um microporous membrane, pack in vials, and freeze-dry according to the freeze-drying process.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com