Process for preparing potassium borofluoride and co-production of white carbon black and sodium fluosilicate
A technology of sodium fluoroborate and potassium fluoroborate is applied in the field of preparing potassium fluoroborate to co-produce white carbon black and sodium fluorosilicate, and can solve the impact of potassium fluoroborate production and cost, lack of market competitiveness, and production process. Complicated and other problems, to achieve the effect of easy promotion and application, low cost, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
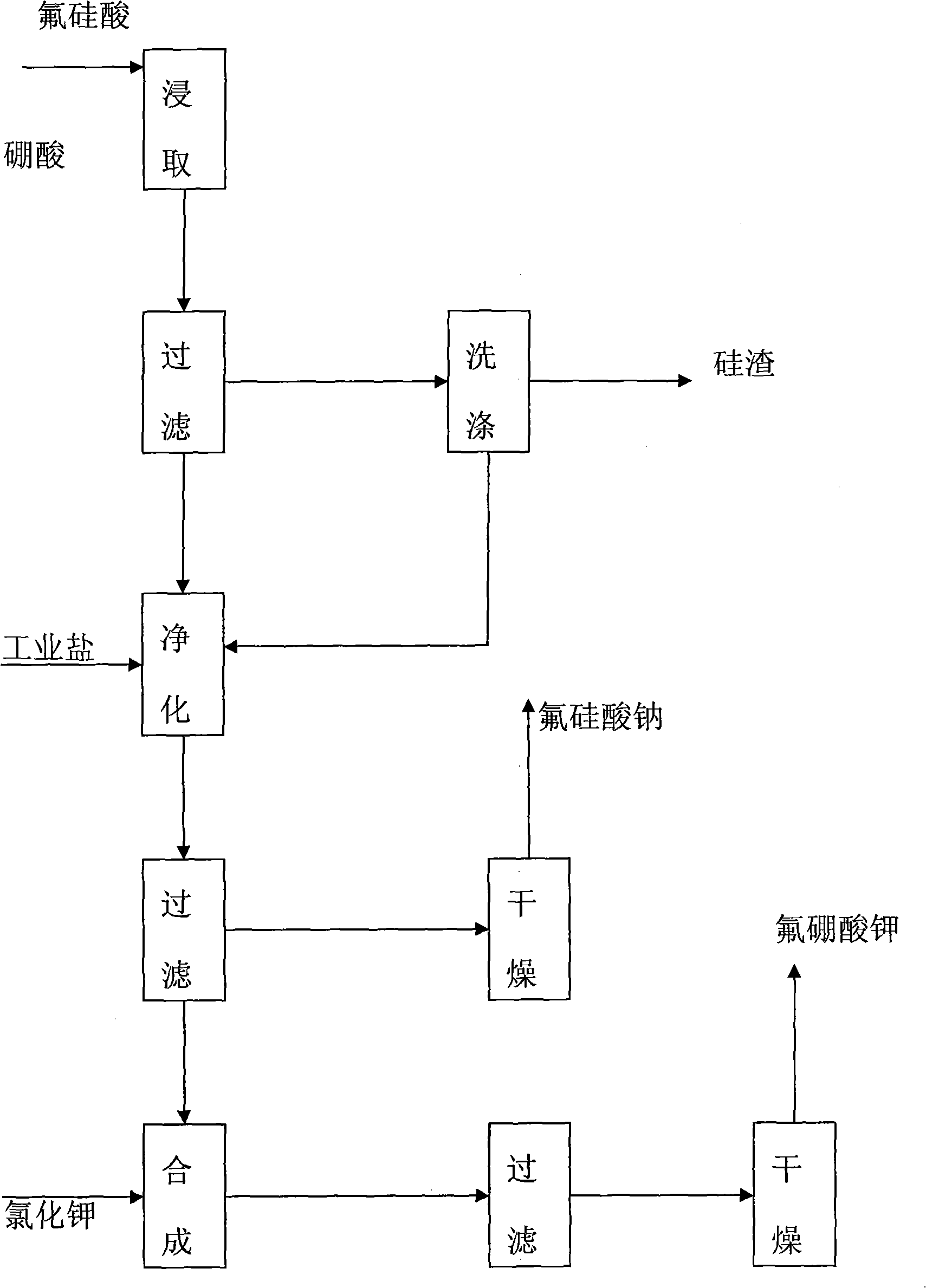
[0025] The preparation method of the co-production of potassium fluoroborate white carbon black and sodium fluorosilicate of the present invention uses fluorosilicic acid, boric acid, potassium chloride and industrial salt as raw materials, specifically comprising the following steps:
[0026] (1) First add fluosilicic acid into the leaching tank, then preheat to 70°C, start stirring, add the theoretical amount of boric acid into the fluosilicic acid solution, seal and continue the reaction for 3 hours, and keep the temperature at 70°C during leaching;
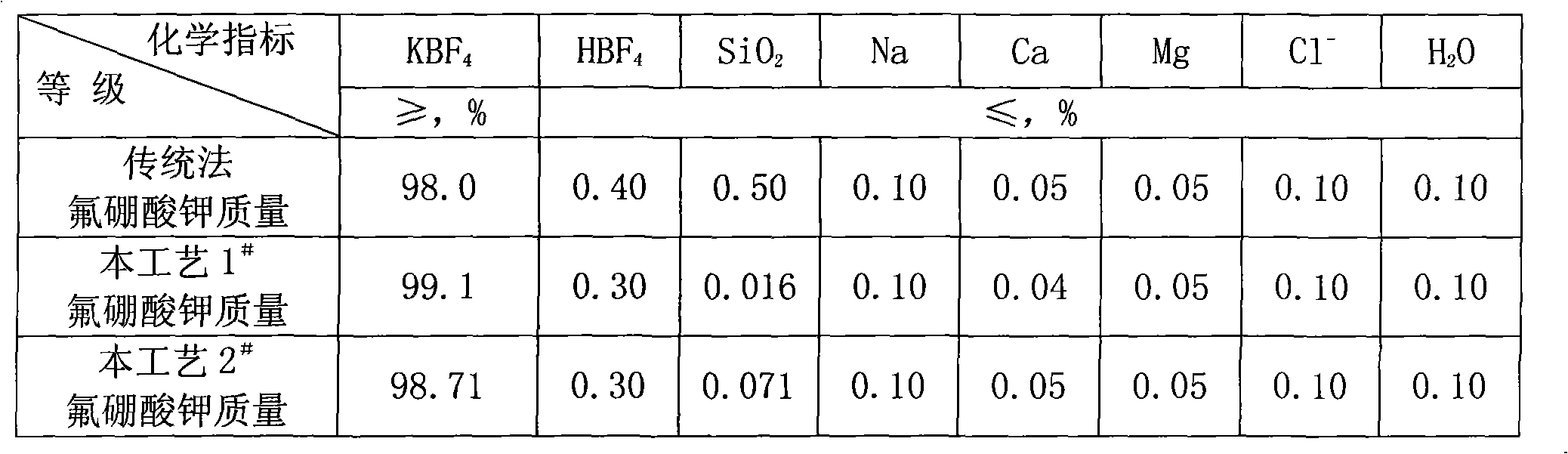
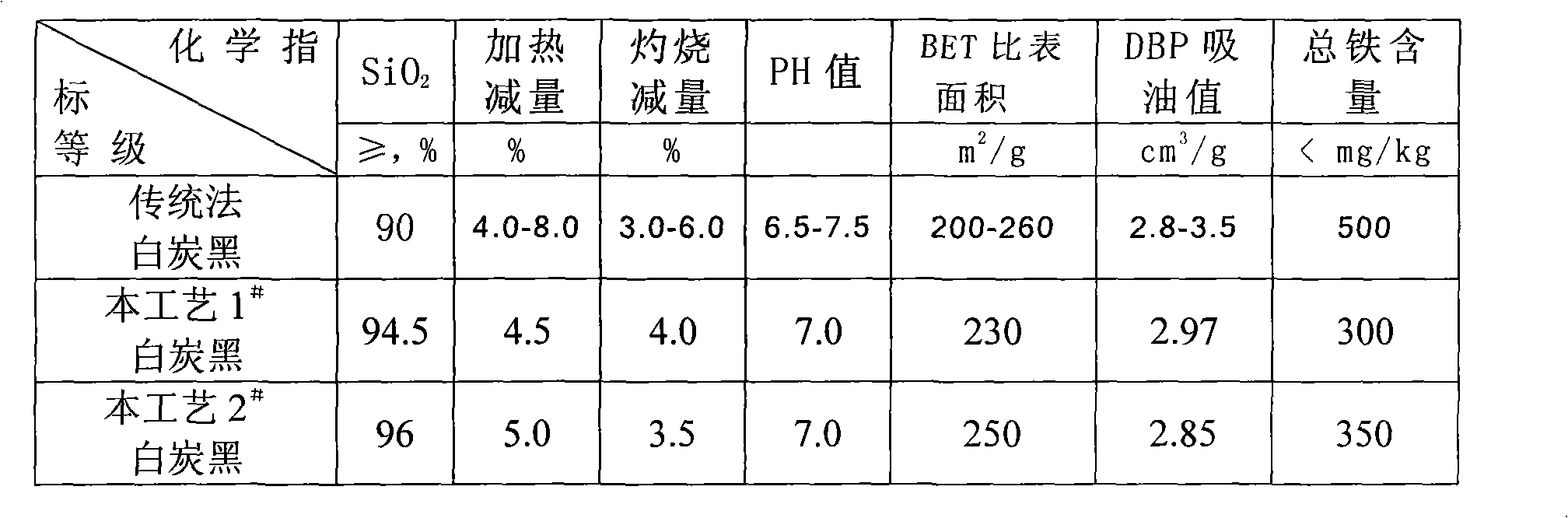
[0027] (2) The qualified fluoboric acid solution obtained by leaching is filtered, and the white carbon black is dried after being concentrated and washed step by step with layered water to obtain a white carbon black product;
[0028] (3) adding industrial salt to the filtrate and the washing liquid for the first time for purification and desiliconization, adding a theoretical amount of industrial salt, and reacting for 10 min...
Embodiment 2
[0034] The preparation method of the co-production of potassium fluoroborate white carbon black and sodium fluorosilicate of the present invention uses fluorosilicic acid, boric acid, potassium chloride and industrial salt as raw materials, specifically comprising the following steps:
[0035] (1) First add fluosilicic acid into the leaching tank, then preheat to 80°C, start stirring, add the theoretical amount of boric acid into the fluosilicic acid solution, seal and continue the reaction for 4 hours, and keep the temperature at 80°C during leaching;
[0036] (2) The qualified fluoboric acid solution obtained by leaching is filtered, and the white carbon black is dried after being concentrated and washed step by step with layered water to obtain a white carbon black product;
[0037] (3) adding industrial salt to the filtrate and the washing liquid for the first time for purification and desiliconization, adding a theoretical amount of industrial salt, and reacting for 20 min...
Embodiment 3
[0043] The preparation method of the co-production of potassium fluoroborate white carbon black and sodium fluorosilicate of the present invention uses fluorosilicic acid, boric acid, potassium chloride and industrial salt as raw materials, specifically comprising the following steps:
[0044] (1) First add fluosilicic acid into the leaching tank, then preheat to 90°C, start stirring, add the theoretical amount of boric acid into the fluosilicic acid solution, seal and continue the reaction for 5 hours, and keep the temperature at 90°C during leaching;
[0045] (2) The qualified fluoboric acid solution obtained by leaching is filtered, and the white carbon black is dried after being concentrated and washed step by step with layered water to obtain a white carbon black product;
[0046] (3) adding industrial salt to the filtrate and the washing liquid for the first time for purification and desiliconization, adding a theoretical amount of industrial salt, and reacting for 30 min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com