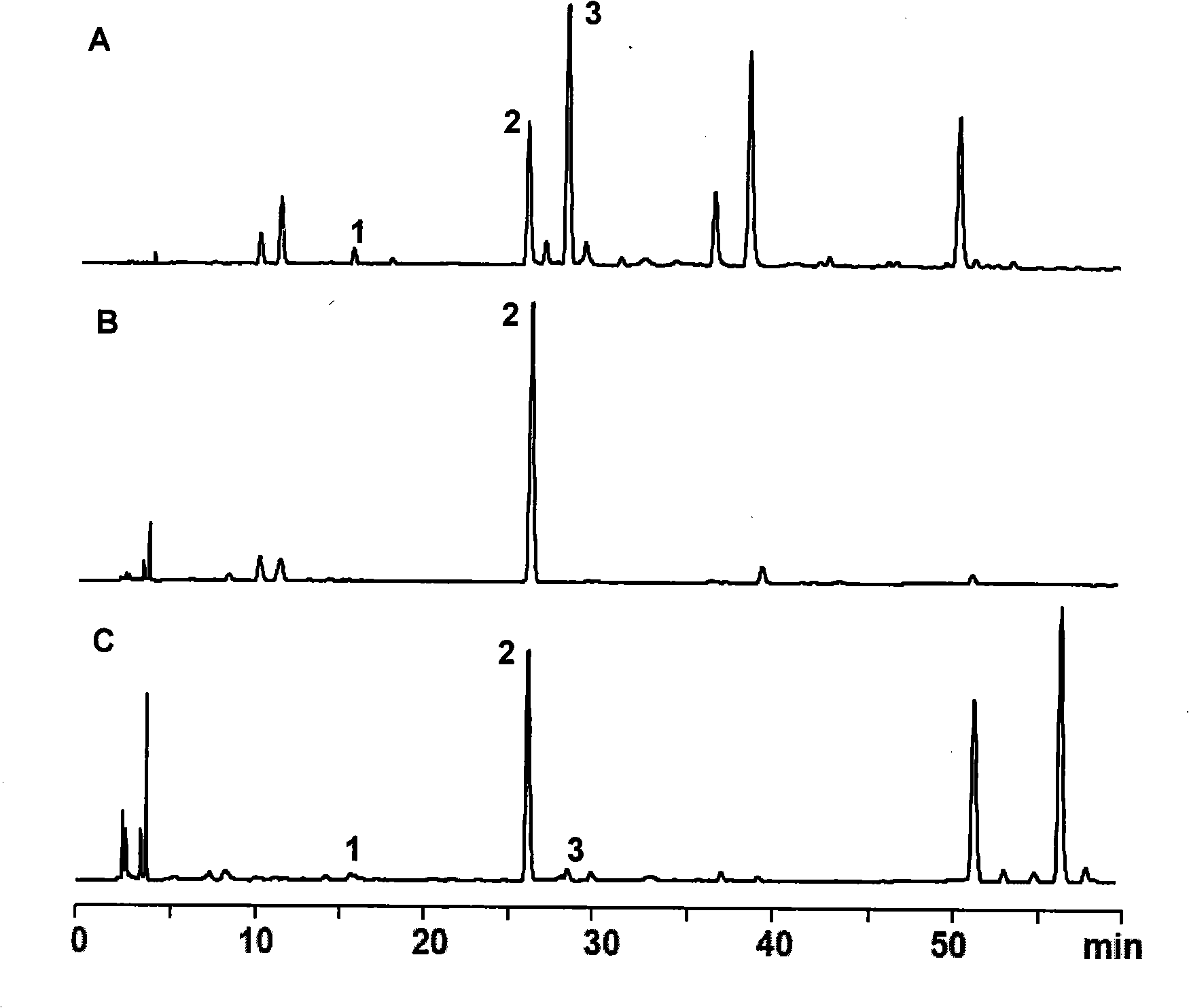
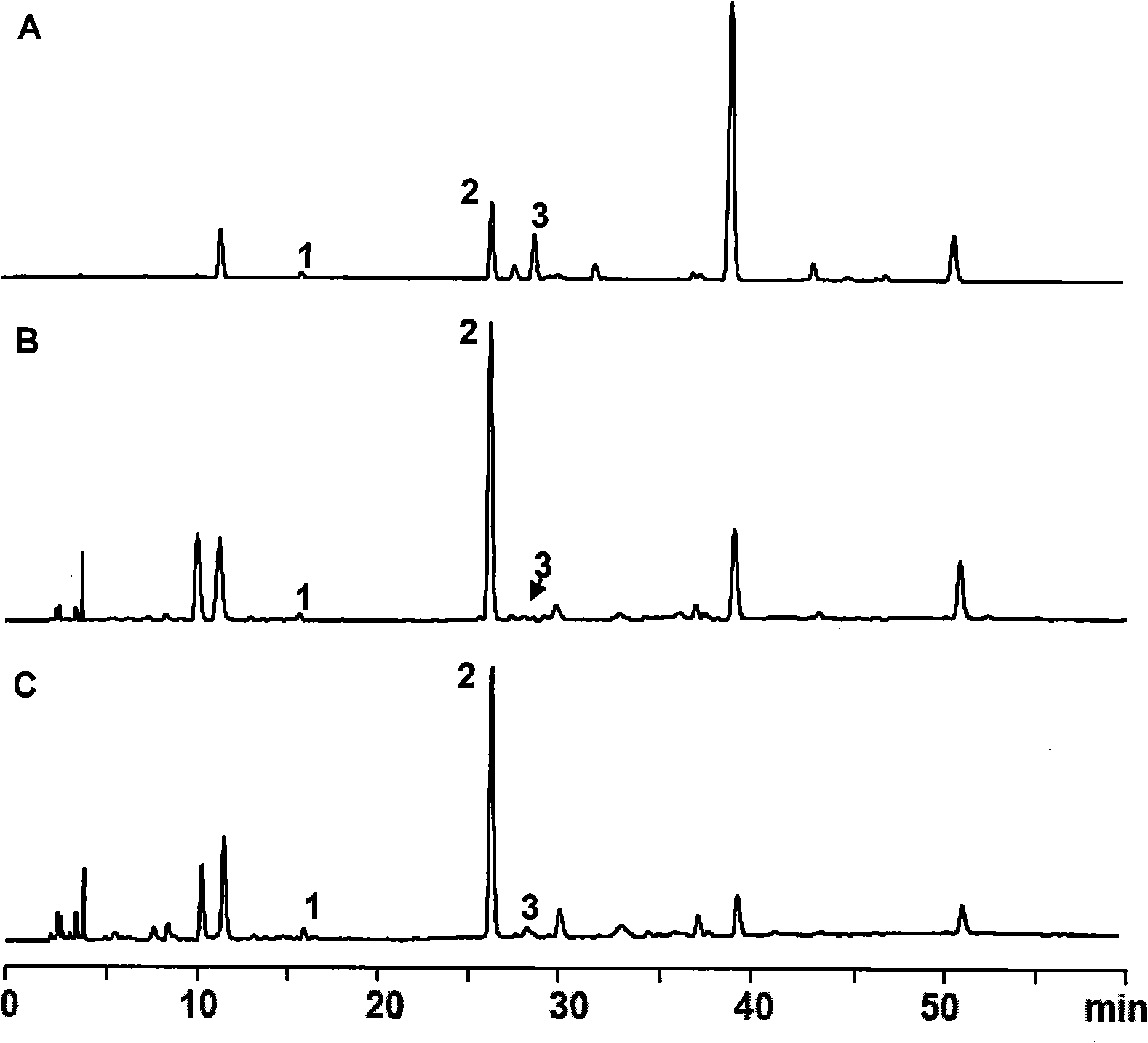
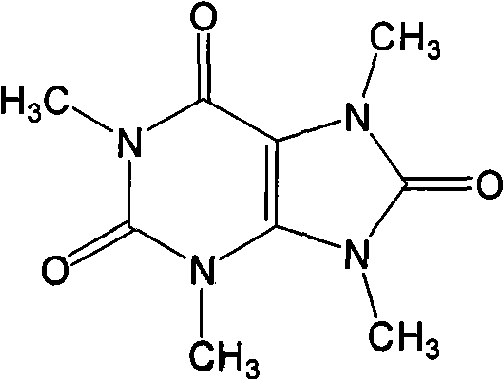
Method for preparing 1,3,7,9-tetramethyl uric acid
A technology of tetramethyluric acid and dichloromethane, applied in the field of chemistry, can solve the problems of difficult industrialized production, high solvent consumption and high process cost, and achieve the effects of simple operation, low solvent consumption and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: adopt the wild bitter tea (Camellia assamica var.kucha) peel of Yunnan origin as raw material:
[0024] One, the preparation of bitter tea peel extract
[0025] Kucha fruit is collected from the wild Kucha tea tree in the place of origin in Yunnan Province. After drying, take out the seeds and crush the peel; weigh 1000g of bitter tea peel powder, add 10000mL of water to extract by ultrasonic (20kHz) at room temperature for 30min, and then heat Decoct for 1 hour, filter, add 8000 mL of water to the filter residue, heat and decoct for 1 hour, combine the two extracts, concentrate under reduced pressure to about 5000 mL, and obtain bitter tea peel extract.
[0026] Two, the preparation of 1,3,7,9-tetramethyluric acid crude product
[0027] The above extracts were extracted with 5000mL chloroform each time, and extracted 3 times in total, the chloroform extracts were combined, and the chloroform was recovered to dryness to obtain 17.2 g of light yellow powde...
Embodiment 2
[0039] Embodiment 2: adopt the wild bitter tea peel of the place of origin in Yunnan as raw material:
[0040] One, the preparation of bitter tea peel extract
[0041] Kucha fruit is collected from the wild Kucha tea tree in the place of origin in Yunnan Province. After drying, take out the seeds and crush the peel; weigh 100g of Kucha peel coarse powder, add 1500mL of water to extract by ultrasonic (80kHz) at room temperature for 10min, and then heat Decoct for 2 hours, filter, and concentrate the filtrate to 400 mL under reduced pressure to obtain bitter tea peel extract.
[0042] Two, the preparation of 1,3,7,9-tetramethyluric acid crude product
[0043] The above extract was extracted with 500 mL of dichloromethane each time, and extracted 3 times in total. The extracts were combined, and the dichloromethane was recovered to dryness to obtain 1.65 g of a light yellow powdery solid, of which 1, 3, 7, and 9 were measured by HPLC. - The content of tetramethyluric acid is 96...
Embodiment 3
[0046] Embodiment 3: adopt the cultivated Kucha pericarp of asexual cutting propagation in the tea garden of Sun Yat-sen University as raw material:
[0047] One, the preparation of bitter tea peel extract
[0048] After picking the Kucha fruit, take out the seeds after drying and crush the pericarp; weigh 100g of the Kucha pericarp powder, add 1000mL of water to extract by ultrasonic (40kHz) at room temperature for 30min, then heat and decoct for 1h, filter, and repeat the decoction for 2 hours. Once, the combined extracts were concentrated under reduced pressure to 500 mL to obtain Kucha pericarp extract.
[0049] Two, the preparation of 1,3,7,9-tetramethyluric acid crude product
[0050] The above extracts were extracted with 500ml of chloroform each time, and extracted twice in total, the chloroform extracts were combined, and the chloroform was recovered to dryness to obtain 1.45 g of light yellow powdery solids, of which 1,3,7,9-tetramethyl The content of base uric aci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com