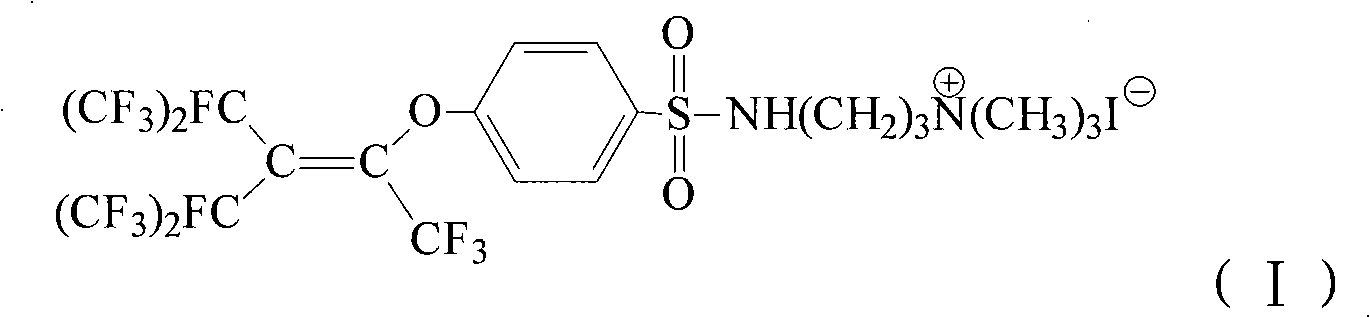
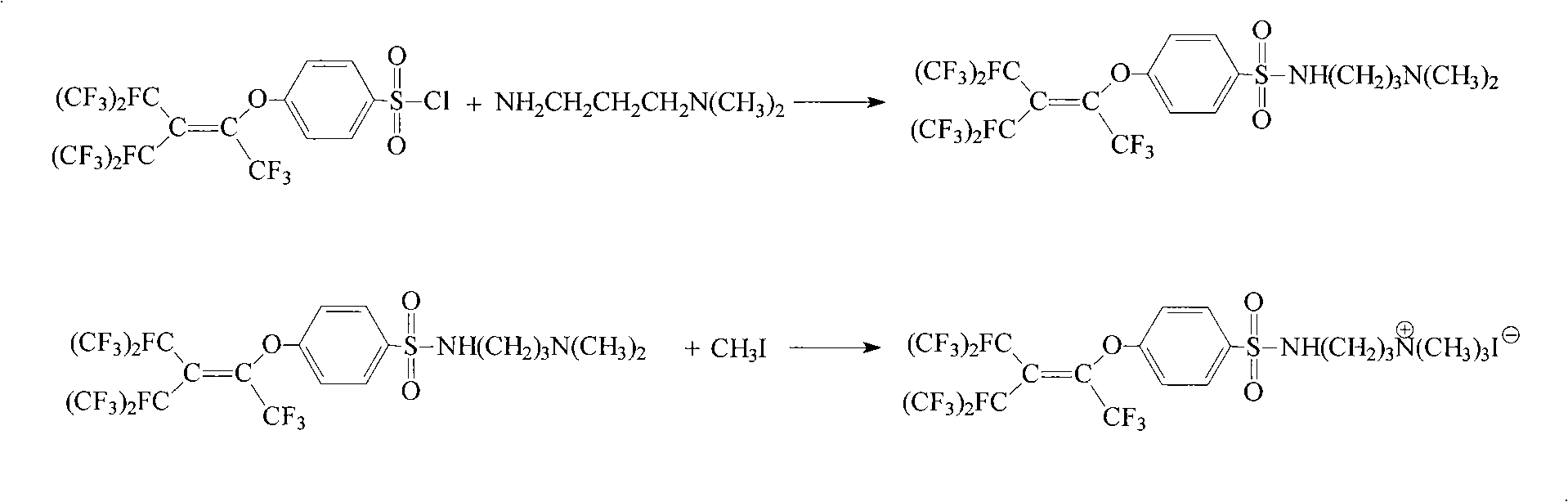Quaternary ammonium salt fluorine surfactant preparing method
A technology of fluorosurfactant and quaternary ammonium salt is applied in the field of preparation of quaternary ammonium salt fluorosurfactant to achieve the effects of high reaction yield, simple synthesis method and low surface tension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 50mL of butanol and 30.3g (0.05mol) of p-perfluorononenyloxybenzenesulfonyl chloride (Lianyungang Taizhuo New Material Co., Ltd.) into a 250mL four-neck flask, stir, raise the temperature to 40°C, and slowly add 5.1g dropwise (0.05mol) N, N-dimethylpropanediamine in butanol (10mL) was stirred for 0.5h, 5.3g (0.05mol) of sodium carbonate powder was added, and stirred at 40°C for 8h. Cool to room temperature, add 50 mL of water and stir, static layering, separation to obtain an organic phase, butanol is distilled off under reduced pressure to obtain a solid. Add the solid and 30 mL of acetonitrile into a 100 mL four-neck flask, heat and stir until the solid dissolves, continue to heat up to 50 °C, slowly add 7.1 g (0.05 mol) of methyl iodide in acetonitrile (10 mL) dropwise, and stir at 50 °C for 3 h . Cool to room temperature, filter to obtain a solid, wash with 10 mL of acetonitrile, then wash with 10 mL of water, filter, and dry in vacuo to obtain N-[3-(p-perfluor...
Embodiment 2
[0033] Add 50mL of cyclohexanone and 30.3g of p-perfluorononenyloxybenzenesulfonyl chloride into a 250mL four-neck flask, stir, raise the temperature to 50°C, and slowly add 6.1 (0.06mol) g of N,N-dimethylpropanedi The amine in cyclohexanone (10 mL) was stirred for 0.5 h, 8.0 g (0.075 mol) of sodium carbonate powder was added, and the reaction was stirred at 50° C. for 6 h. Cool to room temperature, add 50 mL of water and stir, statically separate layers, separate to obtain an organic phase, and distill off cyclohexanone under reduced pressure to obtain a solid. Add the solid obtained above and 30 mL of ethyl acetate into a 100 mL four-neck flask, heat and stir until the solid dissolves. Continue to raise the temperature to 60°C, slowly add 8.5g (0.06mol) methyl iodide in ethyl acetate (10mL) dropwise, and stir at 60°C for 4h. Remove the solvent by distillation under reduced pressure, cool to room temperature, add 10 mL of ethyl acetate to wash the solid, filter and then wash...
Embodiment 3
[0035]Add 50mL of carbon tetrachloride and 30.3g of p-perfluorononenyloxybenzenesulfonyl chloride into a 250mL four-neck flask, stir, raise the temperature to 60°C, and slowly add 7.7g (0.075mol) of N,N-dimethylpropane A solution of diamine in carbon tetrachloride (10 mL) was stirred for 0.5 h, 8.1 g (0.08 mol) of triethylamine was added, and the reaction was stirred for 5 h. Cool to room temperature, add 50mL of water and stir, static layering, separation to obtain the organic phase, carbon tetrachloride is distilled off under reduced pressure to obtain a solid. Into a 100 mL four-neck flask, add the solid obtained above and 30 mL of N,N-dimethylformamide, heat and stir until the solid dissolves. Continue to heat up to 70°C, slowly dropwise add 11.3g (0.08mol) of iodomethane in N,N-dimethylformamide (10mL) solution, and stir at 70°C for 5h. Remove the solvent by distillation under reduced pressure, cool to room temperature, add 10 mL of acetonitrile to wash the solid, filter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com