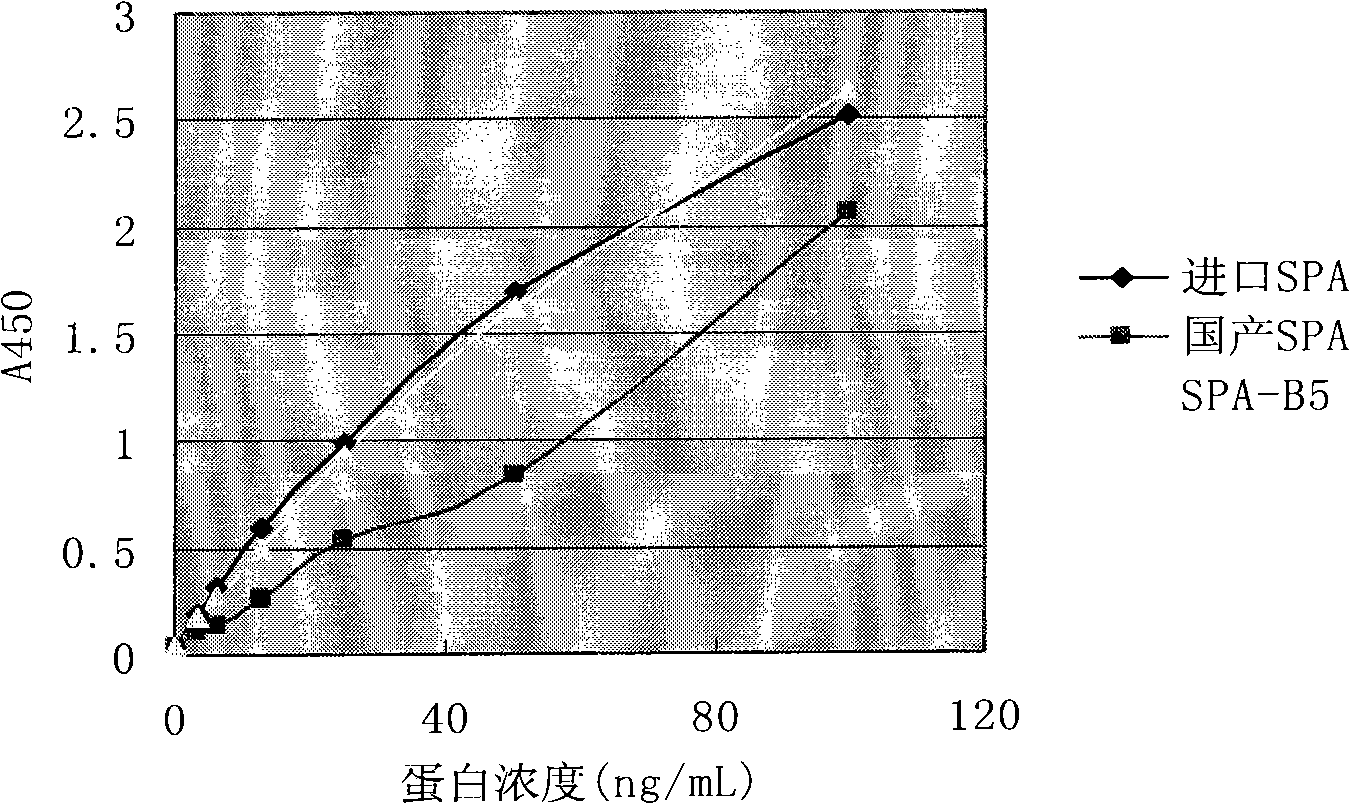
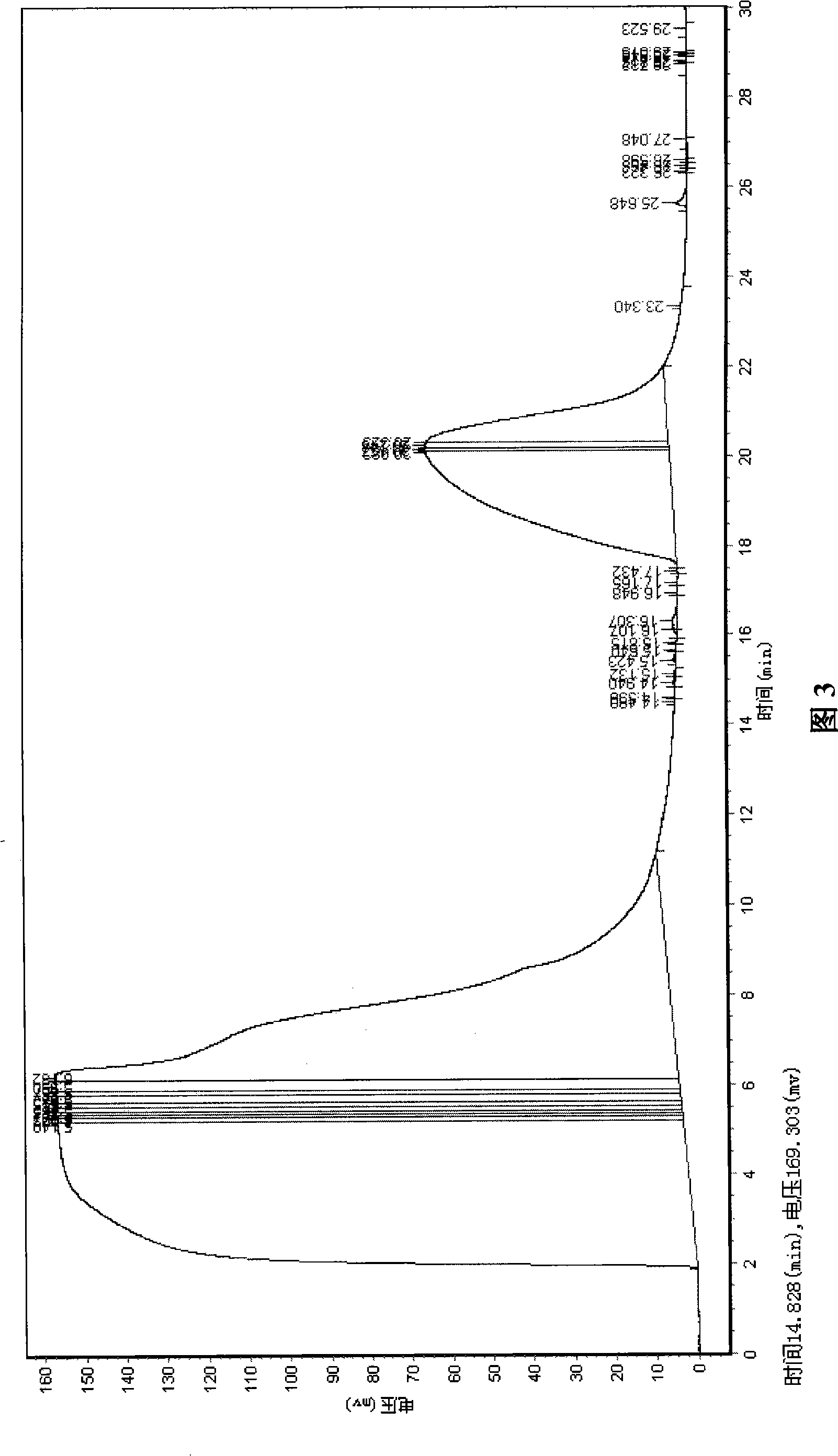
Artificial recombinant penton protein A, construction method and use thereof
A technology of recombinant protein and pentaplex, which is applied in the field of genetic engineering, can solve the problems that cannot meet the application requirements of antibody separation and purification, specific sequence differences, and experimental data cannot be seen.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Acquisition of Recombinant Protein A Gene Monomer
[0032] Design three single-stranded DNA primers, the nucleotide sequences of which are SEQ ID NO.3, SEQ ID NO.4 and SEQ ID NO.5 (see the sequence list), which are synthesized by professional companies and spliced by PCR reactions to obtain recombination The protein A gene monomer contains a BamHI restriction site at the 5' end of the monomer and a HindIII restriction site at the 3' end, and then connects the gene monomer into the vector pQE_TriSystemHisStrep1 by restriction enzyme connection to obtain a recombinant protein The recombinant vector pQE-spa-b of A gene monomer.
Embodiment 2
[0033] Embodiment 2: Acquisition of recombinant protein A gene and construction of expression vector containing recombinant protein A gene Design the first pair of primers:
[0034] CL1: 5'-gggCCATGGGTGcggataacaaattcaacaaag-3'
[0035] CL2: 5'-cccGTCGACttttggtgcttgCgcatc-3'
[0036] Design the second pair of primers:
[0037] CL3: 5'-cccGTCGACaacaaattcaacaaagaac-3'
[0038] CL4: 5'-gggCTCGAGttttggtgcttgCgc-3'
[0039] Use these two pairs of primers to amplify gene monomers respectively, the 5' and 3' ends of the amplified product CL1-2 have NcoI and SalI recognition sites respectively, and the 5' and 3' ends of CL3-4 have SalI respectively and XhoI recognition sites. CL1-2 and CL3-4 were ligated after digestion with SalI to obtain a recombinant protein A gene composed of two gene monomers connected in series, which was named spa-b2. spa-b2 was digested with NcoI and XhoI and ligated with the vector pQE_TriSystemHisStrep1 to obtain the recombinant vector pQE-spa-b2 contain...
Embodiment 3
[0041] Example 3: Construction of Escherichia coli recombinant strain pQE-spa-b5-BL21 (DE3) capable of highly expressing recombinant protein A
[0042] The recombinant vector pQE-spa-b5 was transformed into Escherichia coli BL21(DE3), and the recombinant strain pQE-spa-b5-BL21(DE3) with ampicillin resistance was screened according to conventional methods.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com