Antibody-gold case iron core magnetic nanometer particle synthetic method for cell recognition and separation
A magnetic nanoparticle and nanoparticle technology, applied in the analysis of materials, material magnetic variables, material analysis through optical means, etc., can solve difficult problems such as selectivity reduction, complex synthesis process, long reaction time, etc., and achieve simple detection Intuitive, simple synthesis method, optimized synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
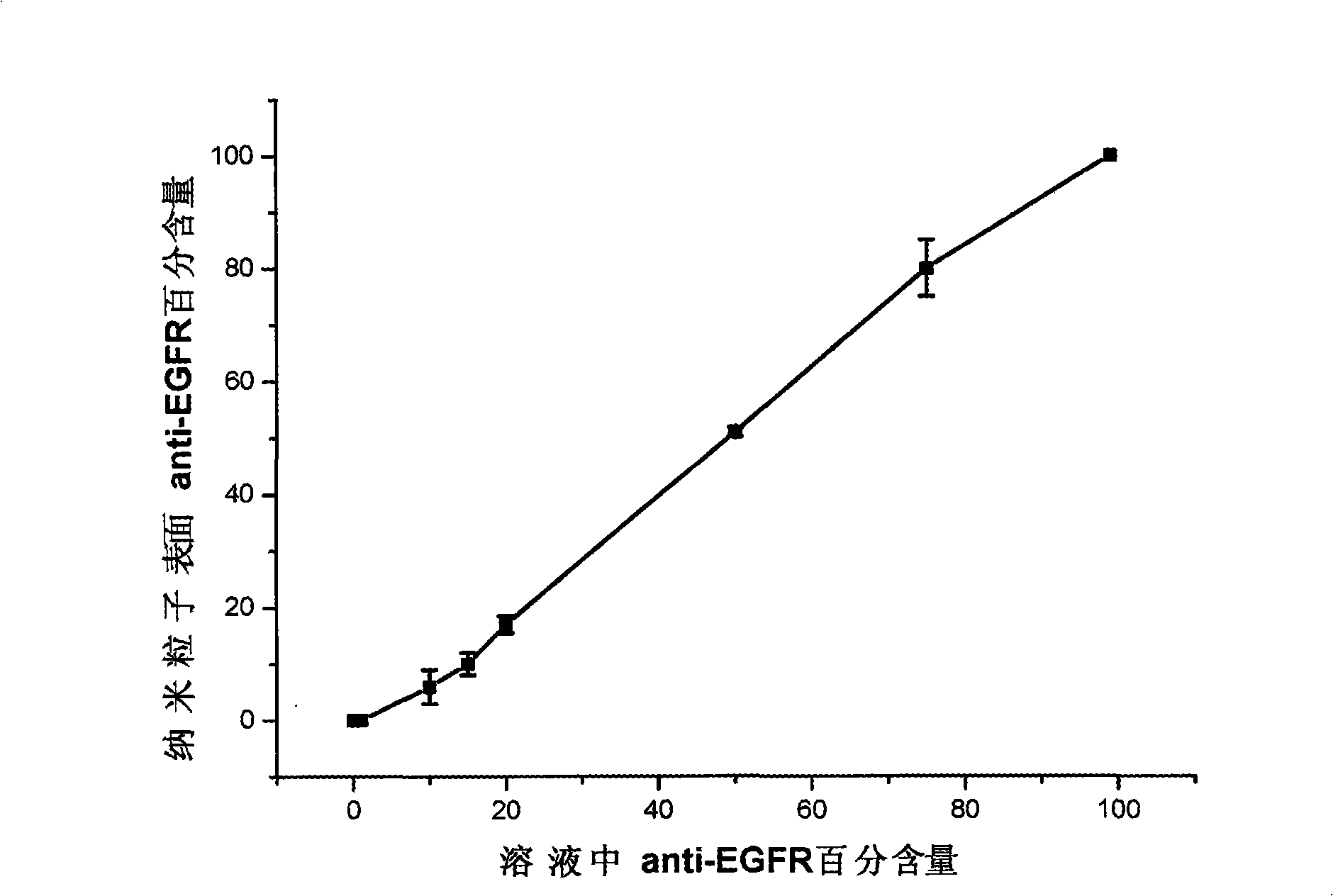
[0028] Preparation Example 1: Synthesis of antibody-gold-shell iron-nuclear magnetic nanoparticles containing 100% epidermal growth factor receptor on the surface for cell recognition and separation, hereinafter referred to as antibody-gold-shell iron-nuclear magnetic nanoparticles
[0029] According to the Lyon method, first utilize 10 milliliters of concentrations to be that the sodium hydroxide of 3.0 mol / liter and 10 milliliters of concentrations all are 10 millimoles / liters ferrous chloride and ferric chloride mixed solution interaction to obtain ferric oxide, further use 10 Milliliter of 0.02 mol / L nitric acid was reacted at 100°C for 1 hour to obtain ferric oxide, and cooled. At room temperature, 10 milliliters of the solution containing the mass concentration of 1% chloroauric acid, 5 mmol / L of sodium citrate and 0.2 mol / L of hydroxylammonium hydrochloride mixed solution was divided 10 times, adding the prepared solution at intervals of 10 minutes each time. Fe2O3 solu...
preparation Embodiment 2
[0032] Preparation Example 2: Synthesis of Antibody-Gold Shell Iron Core Magnetic Nanoparticles Containing 80% Epidermal Growth Factor Receptor Antibody on the Surface
[0033] According to the Lyon method, first utilize 10 milliliters of concentrations to be that the sodium hydroxide of 3.0 mol / liter and 10 milliliters of concentrations all are 10 millimoles / liters of ferrous chloride and ferric chloride mixed solution interaction to obtain ferric oxide, further use 10 Milliliter of 0.02 mol / L nitric acid was reacted at 100°C for 1 hour to obtain ferric oxide, and cooled. At room temperature, 10 milliliters of solutions containing the mass concentration of 1% chloroauric acid, 5 mmol / L of sodium citrate and 0.2 mol / L of hydroxylammonium hydrochloride were divided into 10 times, adding the prepared solution at intervals of 10 minutes each time. Fe2O3 solution to obtain gold-shell iron-core magnetic nanoparticles. Its particle size is 60 nm.
[0034] Dissolve cystylalanylleuc...
preparation Embodiment 3
[0036] Preparation Example 3: Synthesis of Antibody-Gold Shell Iron Core Magnetic Nanoparticles Containing 50% Epidermal Growth Factor Receptor Antibody on the Surface
[0037] According to the Lyon method, first utilize 10 milliliters of concentrations to be that the sodium hydroxide of 3.0 mol / liter and 10 milliliters of concentrations all are 10 millimoles / liters of ferrous chloride and ferric chloride mixed solution interaction to obtain ferric oxide, further use 10 Milliliter of 0.02 mol / L nitric acid was reacted at 100°C for 1 hour to obtain ferric oxide, and cooled. At room temperature, 10 milliliters of solutions containing the mass concentration of 1% chloroauric acid, 5 mmol / L of sodium citrate and 0.2 mol / L of hydroxylammonium hydrochloride were divided into 10 times, adding the prepared solution at intervals of 10 minutes each time. Fe2O3 solution to obtain gold-shell iron-core magnetic nanoparticles. Its particle size is 60 nm.
[0038] Dissolve cystylalanylleuc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com