Process for synthesizing hydroxylapatite
A technology for synthesizing hydroxyapatite and hydrothermal synthesis, which is applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of harsh reaction conditions, high acquisition cost, and limited quantity, and achieve fast reaction speed, The effect of abundant resources and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
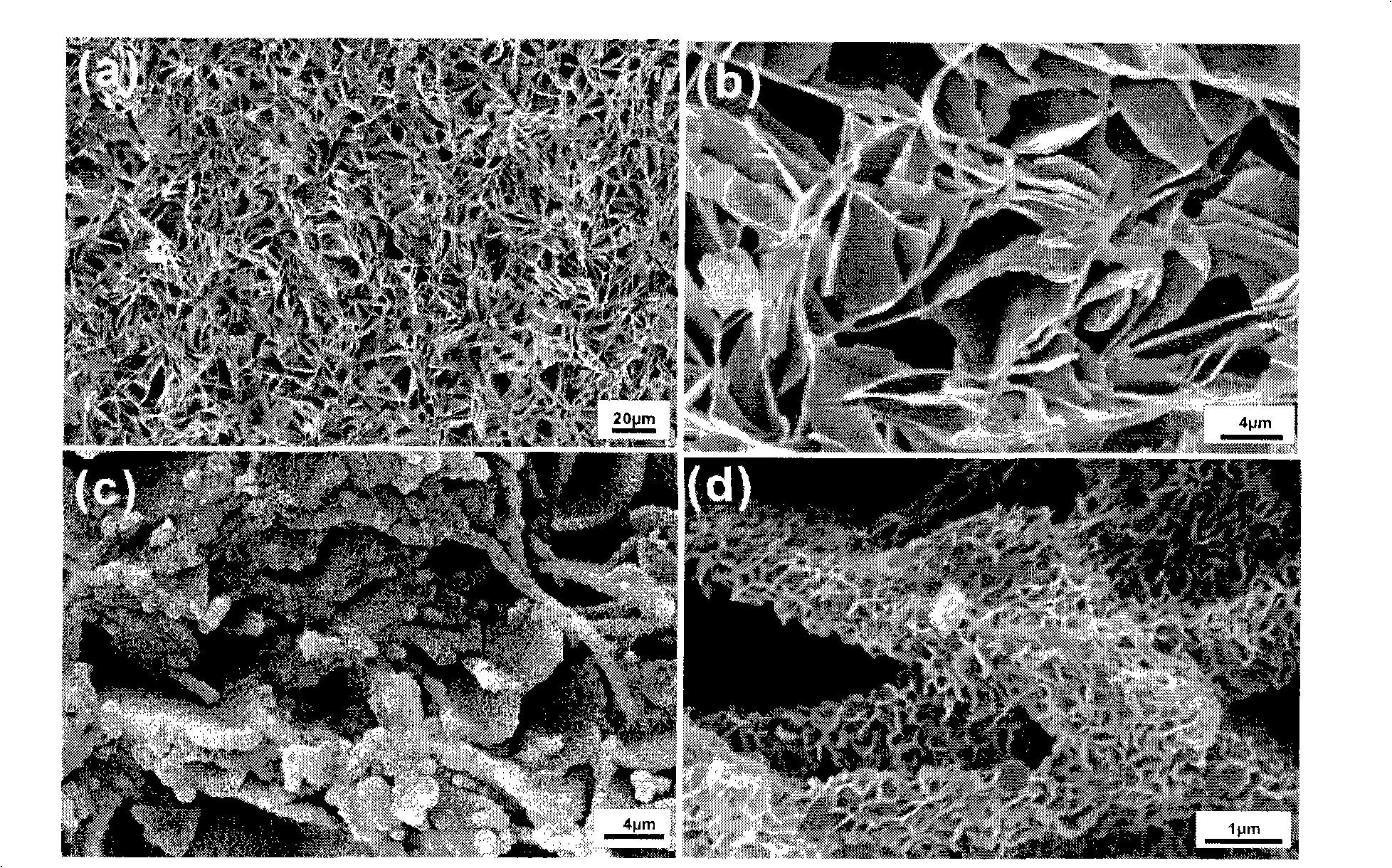
Image
Examples
Embodiment 1
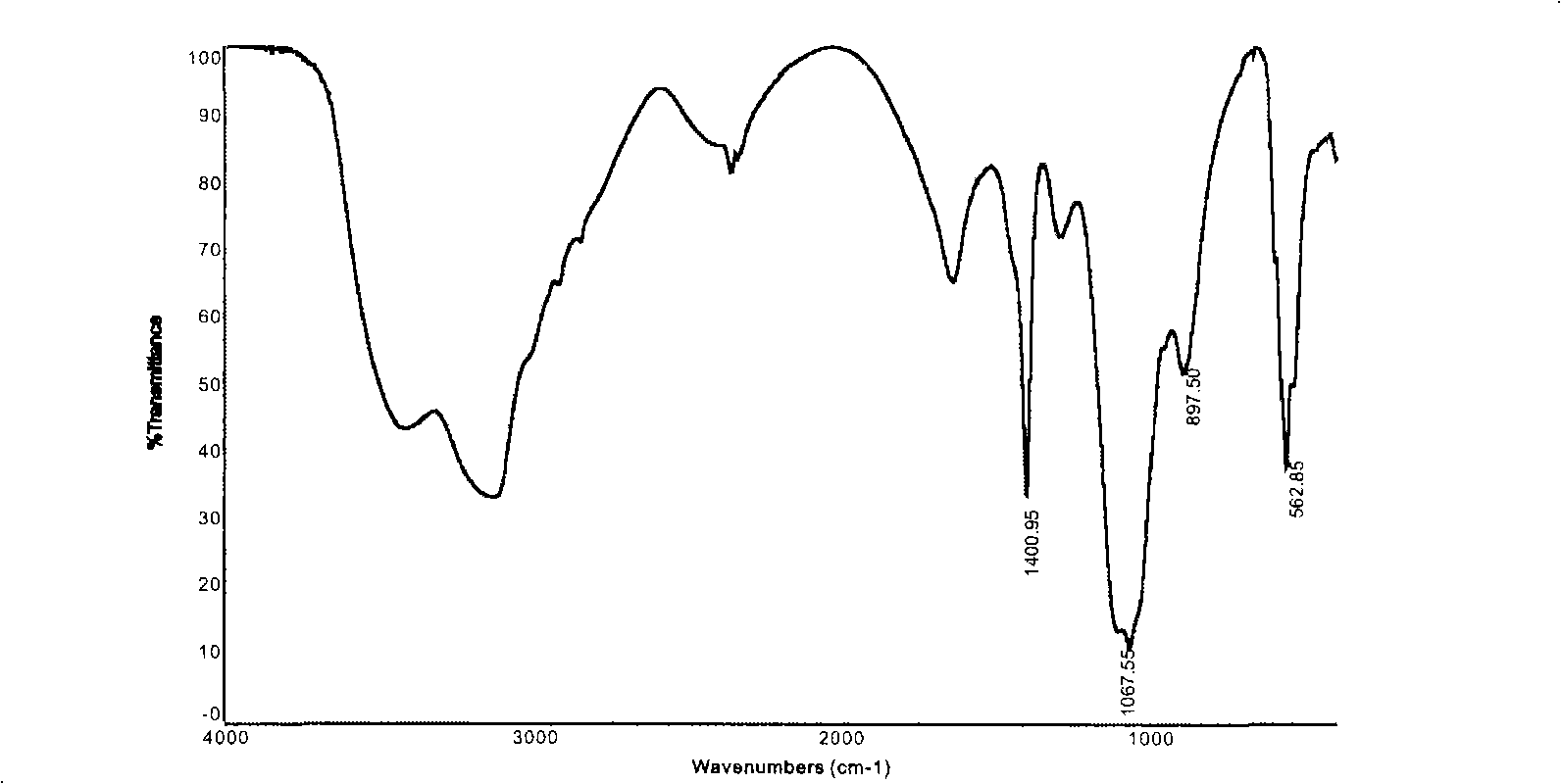
[0039] Weigh 1.0g of the dried chalk layer, put it into a 50ml reaction test tube with 1.0g of diammonium hydrogen phosphate, add about 50ml of water to completely dissolve the diammonium hydrogen phosphate, and then plug the mouth of the test tube with medical cotton. Stand in the air at room temperature 30°C, stop the reaction after 120h, take out the sample and wash it thoroughly, dry it at 80°C and grind it into powder for FTIR test, as shown in Figure-2, from the infrared spectrum in Figure-2, it can be seen that the chalk layer (calcium carbonate calcite) and diammonium hydrogen phosphate aqueous solution at room temperature 30 ℃, the characteristic absorption peak of hydroxyapatite does not appear after 120h (υ 1 960.5cm -1 ; 3 1035.4cm -1 , 1091cm -1 ; 4 564.4cm -1 , 603cm -1 ), that is, no hydroxyapatite is produced.
Embodiment 2
[0041] Take by weighing 1.0g (0.01 mole) dry chalk layer, put into the reaction test tube that has the reflux condenser tube of 50ml with 1.0g (0.0076 mole) diammonium hydrogen phosphate, add 50ml water, make diammonium hydrogen phosphate Dissolve completely, and then place it in a constant temperature water bath at 60°C and heat it. After 48 hours of reaction, samples were taken, washed and dried, and the test results of FTIR and XRD were shown in Figure-3 and Figure-4 respectively.
[0042] It can be seen from Figure-3 that the chalk layer reacted with diammonium hydrogen phosphate aqueous solution at 60°C for 48 hours to convert hydroxyapatite, but the CO of calcite calcium carbonate 3 2- Characteristic absorption peak (879.35cm -1 ,712.75cm -1 ) still exists, indicating that there is a surplus of calcite. The same conclusion can also be drawn from the XRD analysis of the product (Figure-4), and it can be seen that there is more calcite remaining (the relative intensity...
Embodiment 3
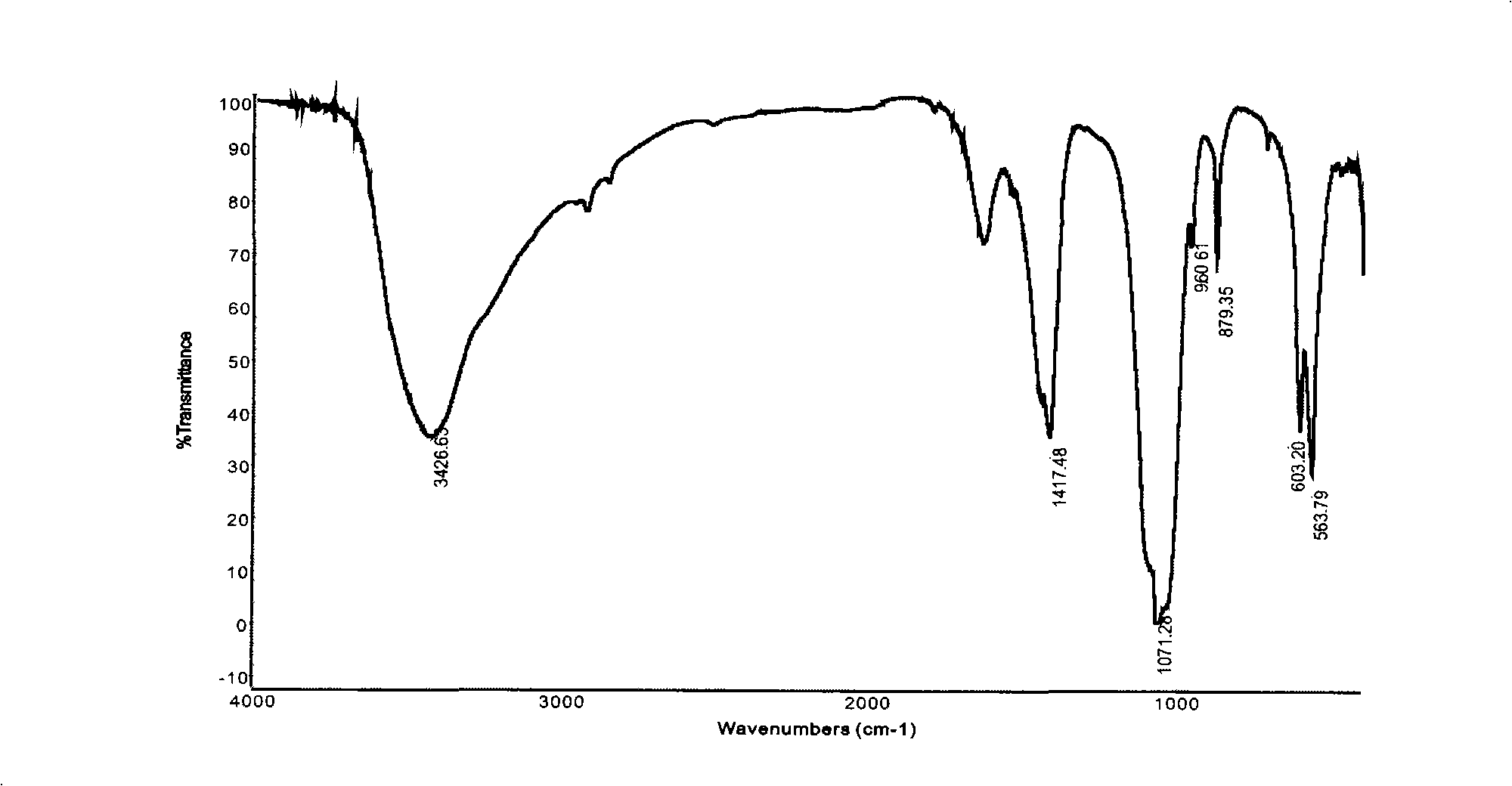
[0044]Take by weighing two parts of each 1.0g dry chalk layer, put into the reaction test tube that has the reflux condensation tube of 50ml with 1.0g diammonium hydrogen phosphate respectively, add 50ml water, make diammonium hydrogen phosphate dissolve completely, then Placed in a 75°C constant temperature water bath for heating. Take one of the samples in 24 hours, wash, dry, and grind for FTIR test as shown in Figure-5. The reaction of another sample stopped after 48 hours, and the sample was washed and dried for XRD analysis as shown in Figure-6.
[0045] It can be seen from Figure-5 that the characteristic absorption peak of hydroxyapatite (υ 1 960cm -1 ; 3 1035cm -1 , 1090cm -1 ; 4 564cm -1 , 603cm -1 ), indicating the generation of hydroxyapatite. From the XRD pattern in Figure-6, it can be seen that after 48 hours of reaction, the calcite is still partially unreacted (the weaker peaks of calcite and tricalcium phosphate are not marked).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com