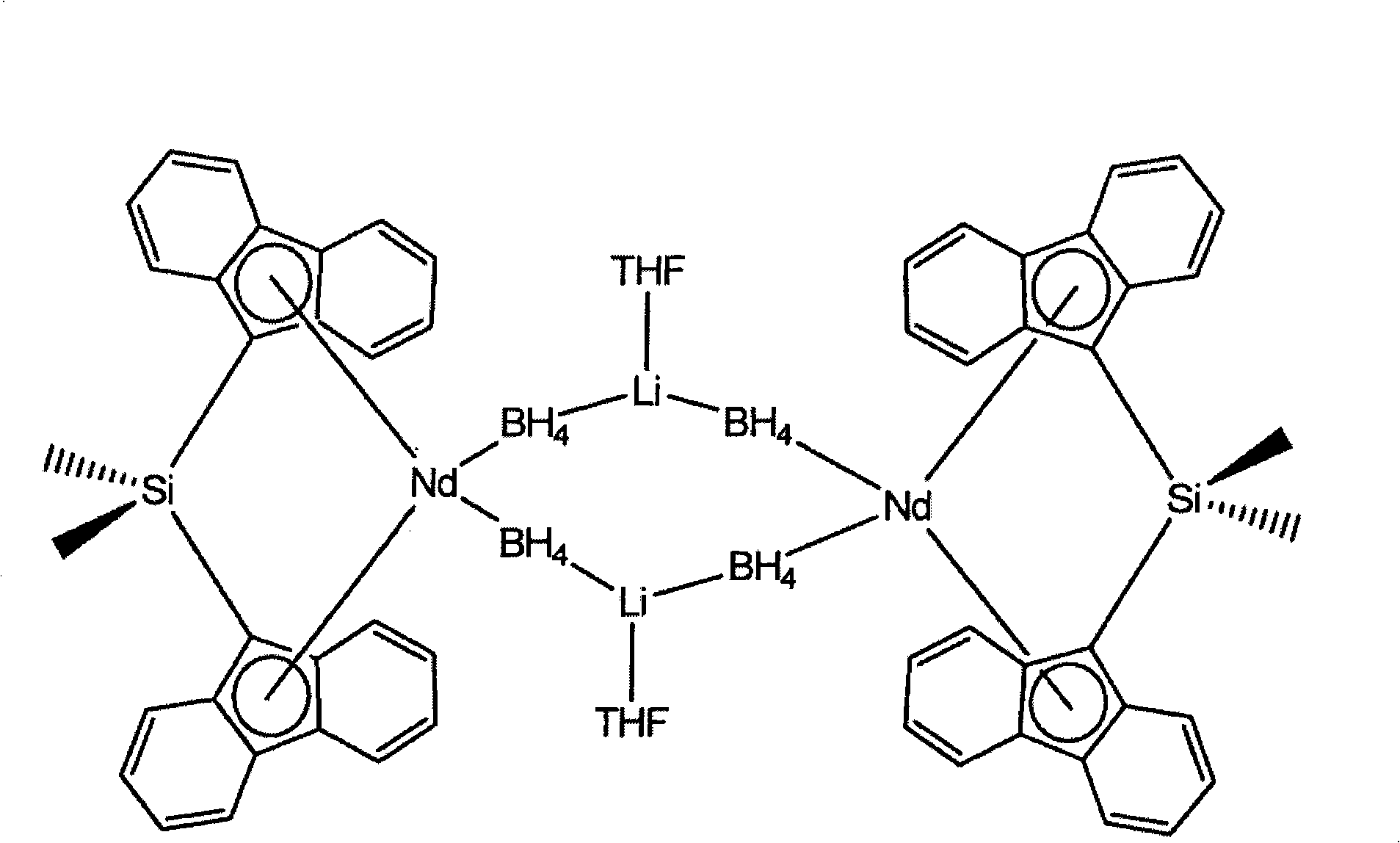
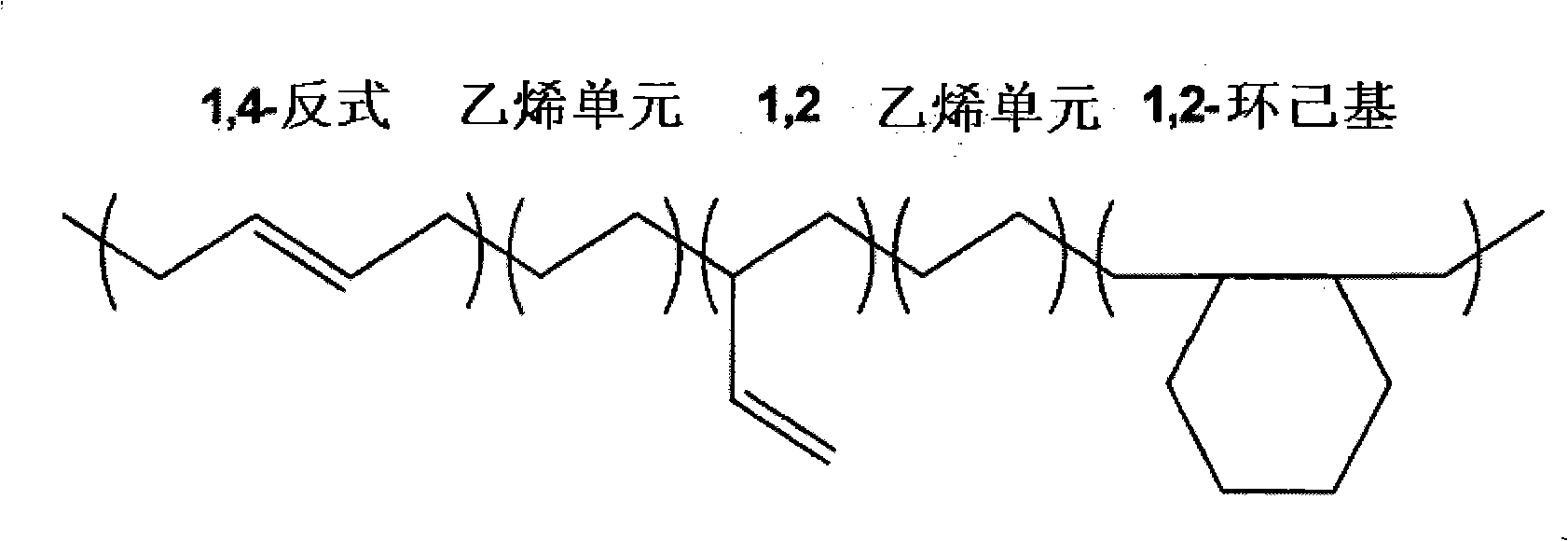
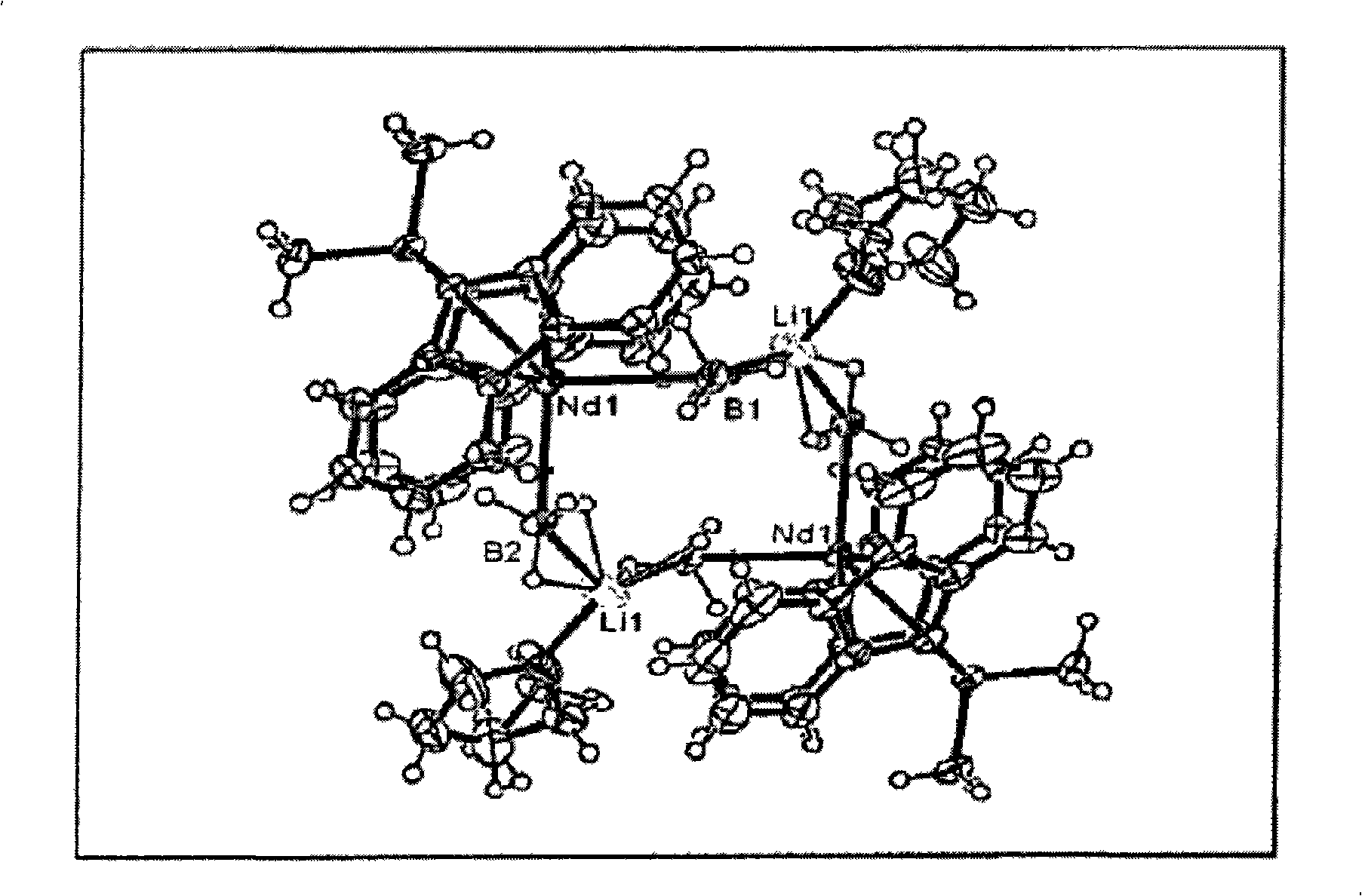
Borohydride metallocene complex of a lanthanide, catalytic system including said complex, polymerization method using same and ethylene/butadiene copolymer obtained using said method
A metallocene complex, lanthanide technology, applied to lanthanide borohydride metallocene complexes, catalytic systems comprising said complexes, polymerization using the same complexes and using said obtained ethylene- In the field of butadiene copolymers, it is possible to solve problems such as the description of copolymerization of olefins and conjugated dienes without the use of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0084] All of the following examples were carried out under argon atmosphere with pre-drying of the used solvents by reaction with sodium followed by distillation or passing through 3 Angstrom molecular sieves while flushing with argon.
[0085] All the metallocene complexes synthesized below are at 22°C in d 8 - Passed at 300MHz using a "Bruker DRX 300" spectrometer in THF 1 The analysis was carried out by H NMR and, furthermore, for the hydroboration complexes according to the present invention, by the X-ray diffraction technique described in Additional Annex 1.
[0086] The microstructure of each copolymer obtained in these examples was obtained by 1 H NMR and 13 C NMR technique determination. For this, a "Bruker DRX 400" spectrometer was used at 400 MHz for 1 H NMR technique at 100.6MHz for 13 C NMR techniques. Spectra were collected at a temperature of 363K using a 5 mm "QNP" probe. A tetrachloroethylene / perdeuterated benzene mixture (2:1 by volume) was used as sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com