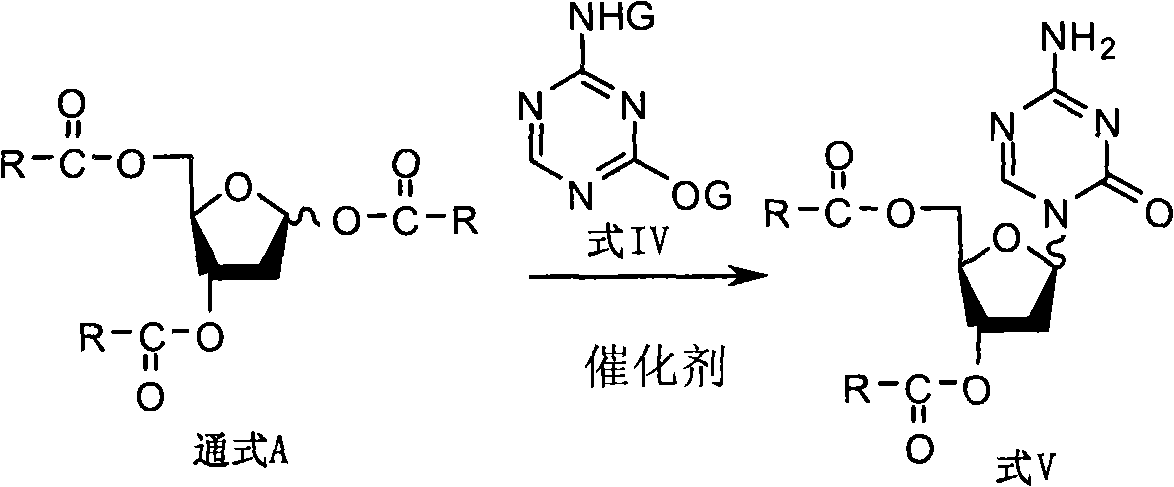
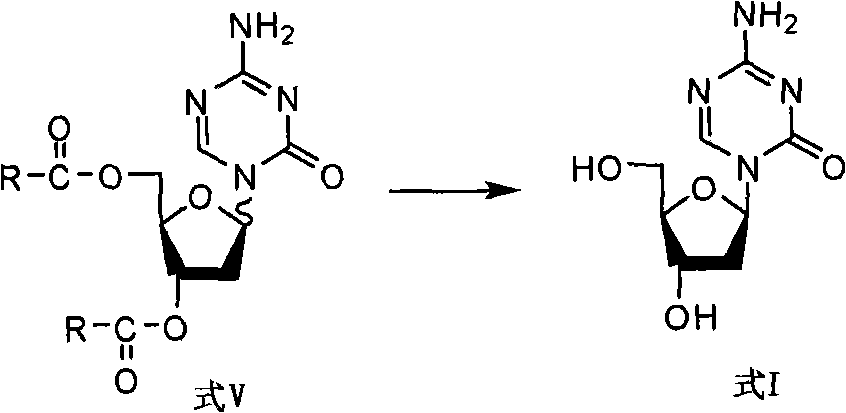
2-deoxy-d-ribose derivates, preparation method and use thereof
A technology for derivatives and uses, applied in the field of derivatives of 2-deoxy-D-ribose and their preparation, can solve the problems of danger, incomplete reaction, hydrolysis and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
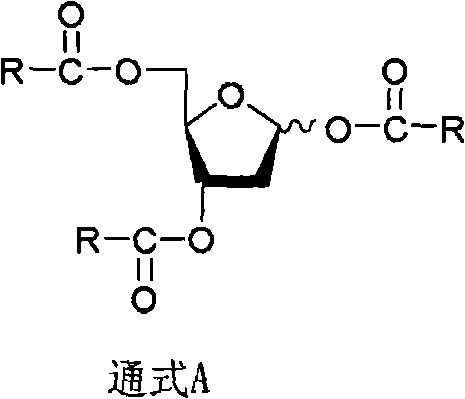
[0089] The invention provides a preparation method of the compound of the general formula A, which comprises substituting and protecting the three hydroxyl groups at positions 1, 3 and 5 of 2-deoxy-D-ribose (formula II) through acetylation to obtain the compound of the general formula A.
[0090]
[0091] Formula II General Formula A
[0092] The compound of formula II is mixed with a suitable solvent containing a deacidifying agent, and the substituted acetylation reagent is slowly added dropwise at -50°C to 30°C (preferably -10°C to 10°C). °C-30°C) for 15-45 hours (preferably 20-30 hours), to obtain the compound of general formula A. Wherein the molar ratio of the substituted acetylating agent to the compound of formula II is 1-9:1 (preferably 3-6:1, more preferably 3-4:1).
[0093] The suitable solvent containing deacidification agent includes: tertiary amine (pyridine, lower alkane substituted pyridine, triethylamine, etc.), or a mixed system of various aprotic polar s...
Embodiment 1
[0120] 1,3,5-tris(methoxyacetyl)-2-deoxy-D-ribose (general formula A, R=CH 3 OCH 2 ) preparation
[0121] 2-Deoxy-D-ribose (II) (20g) was dissolved in pyridine (60ml), cooled to 0°C, methoxyacetic anhydride (75ml) was slowly added dropwise, and the mixture was stirred at room temperature for 30 hours. Add dichloromethane for dilution, adjust the pH value to neutral with saturated aqueous sodium bicarbonate solution, wash the organic phase successively with 2mol / L hydrochloric acid, saturated aqueous sodium bicarbonate solution, and saturated brine, dry over anhydrous sodium sulfate, and concentrate under reduced pressure to obtain the Formula A compound (R=CH 3 OCH 2 ), as light yellow syrup (56g). Without purification, it was directly put into the next reaction. ESI-MS(m / z)373(M+Na) + .
Embodiment 2
[0123] 1,3,5-tris(chloroacetyl)-2-deoxy-D-ribose (general formula A, R=ClCH 2 ) preparation
[0124] 2-Deoxy-D-ribose (II) (20g) was dissolved in pyridine (60ml), cooled to -5°C, chloroacetyl chloride (38ml) was slowly added dropwise, and the mixture was stirred at room temperature for 24 hours. Add dichloromethane for dilution, adjust the pH value to neutral with saturated aqueous sodium bicarbonate solution, wash the organic phase successively with 2mol / L hydrochloric acid, saturated aqueous sodium bicarbonate solution, and saturated brine, dry over anhydrous sodium sulfate, and concentrate under reduced pressure to obtain the Formula A compound (R=ClCH 2 ) is pale yellow syrup (54g). Without purification, it was directly put into the next reaction. ESI-MS(m / z)385(M+Na) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com