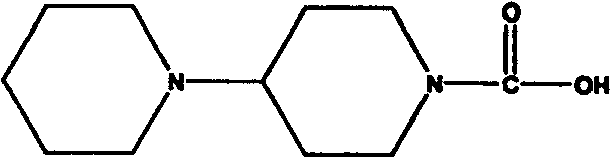
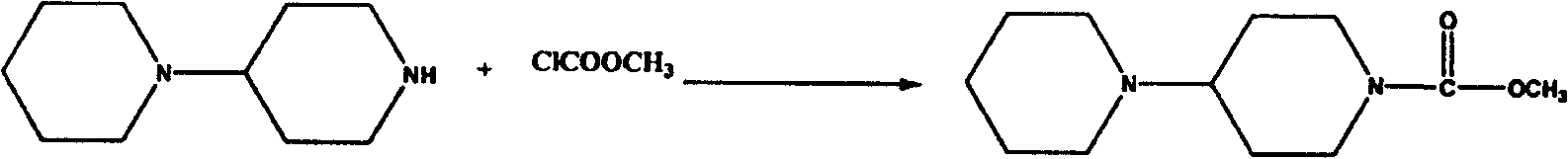Preparation for midbody of Irinotecan and preparation for Irinotecan
A technology for irinotecan and intermediates, which is applied in the field of synthesis of camptothecin-related compounds, can solve the problems of unfavorable high-purity target substances, large solvent consumption, long chromatography cycle, etc., and achieves the advantages of reducing treatment costs and reducing impurities. content, the effect of easy recrystallization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation method of the intermediate of irinotecan
[0036] 1. Acylation step:
[0037] 20 g (134.2 mmol) of piperidinyl piperidine was dissolved in 100 ml of dichloromethane, then 35.6 g of sodium carbonate was added, 13.9 g (147.6 mmol) of methyl chloroformate was added dropwise at room temperature, and the reaction was carried out at room temperature for 24 hours . Washed with water, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered and concentrated to obtain 26 g of the target product, yield: 93.6%.
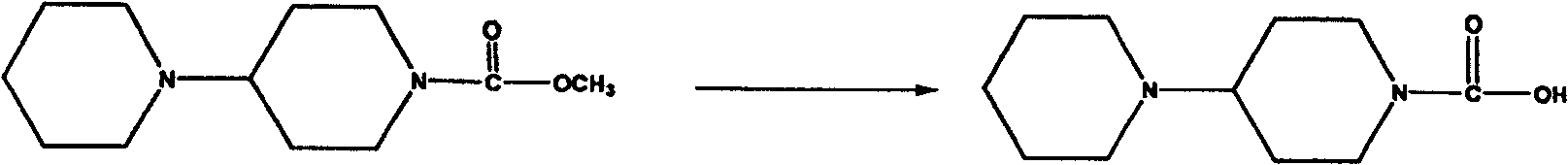
[0038] 2. Hydrolysis step:
[0039] 26 grams (125.6 mmol) obtained in the above steps were dissolved in 120 milliliters of methanol, then 30 milliliters of an aqueous solution of 8.5 grams of sodium hydroxide (213.5 mmol) was added, and the reaction was heated to reflux for about 5 hours. After the reaction was completed, methanol was evaporated under reduced pressure, and Take 50 ml of water, wash with 30×3 ml of ethy...
Embodiment 2
[0040] Embodiment 2: the method for preparing irinotecan using intermediate
[0041] Condensation step: 44 grams (112.3 mmol) of 7-ethyl-10-hydroxycamptothecin, 24.9 grams (118 mmol) of piperidinyl pipecolic acid were added to a 500-ml three-necked bottle, 200 milliliters of pyridine was added, dichloro 150 ml of methane, stirred and cooled to -5°C, 1 g of DMAP, 25.2 g (123.5 mmol) of DCC, kept at this temperature until the reaction was completed, filtered, washed the filtrate with saturated sodium bicarbonate, washed with saturated brine, and dried over anhydrous magnesium sulfate , filtered, and concentrated to obtain 59 g of the target product irinotecan, yield: 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com