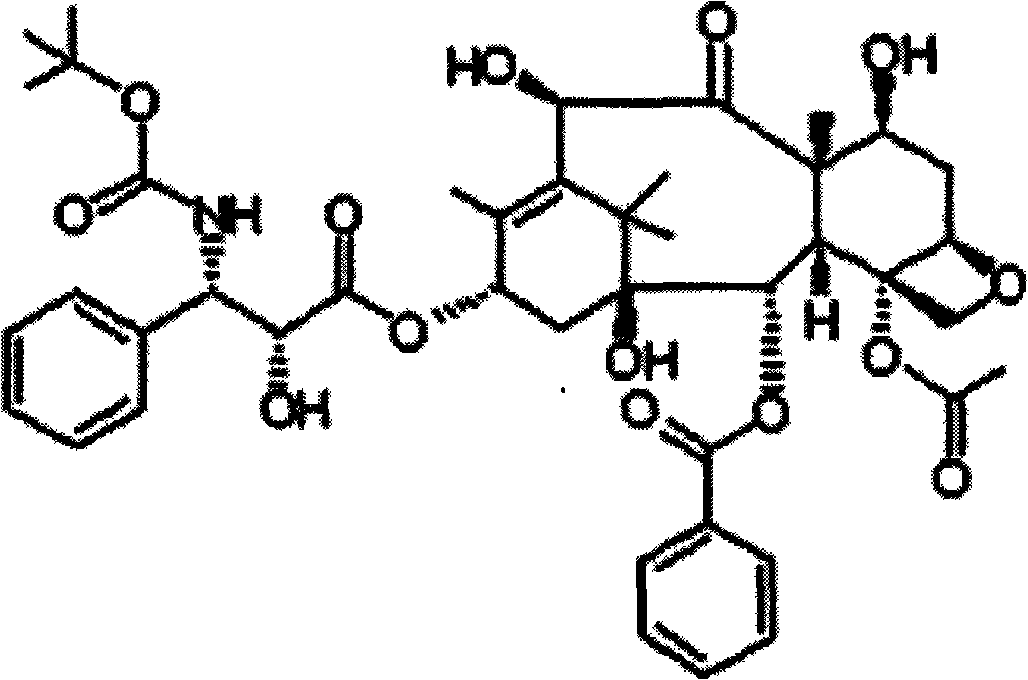
Docetaxel long-circulation formulation
A docetaxel and long-circulation technology, applied in the field of long-circulation preparations, can solve the problems of inconvenient clinical medication, high viscosity of Tween-80, etc., achieve strong inhibitory effect, strong hydrophilicity, and avoid phagocytosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
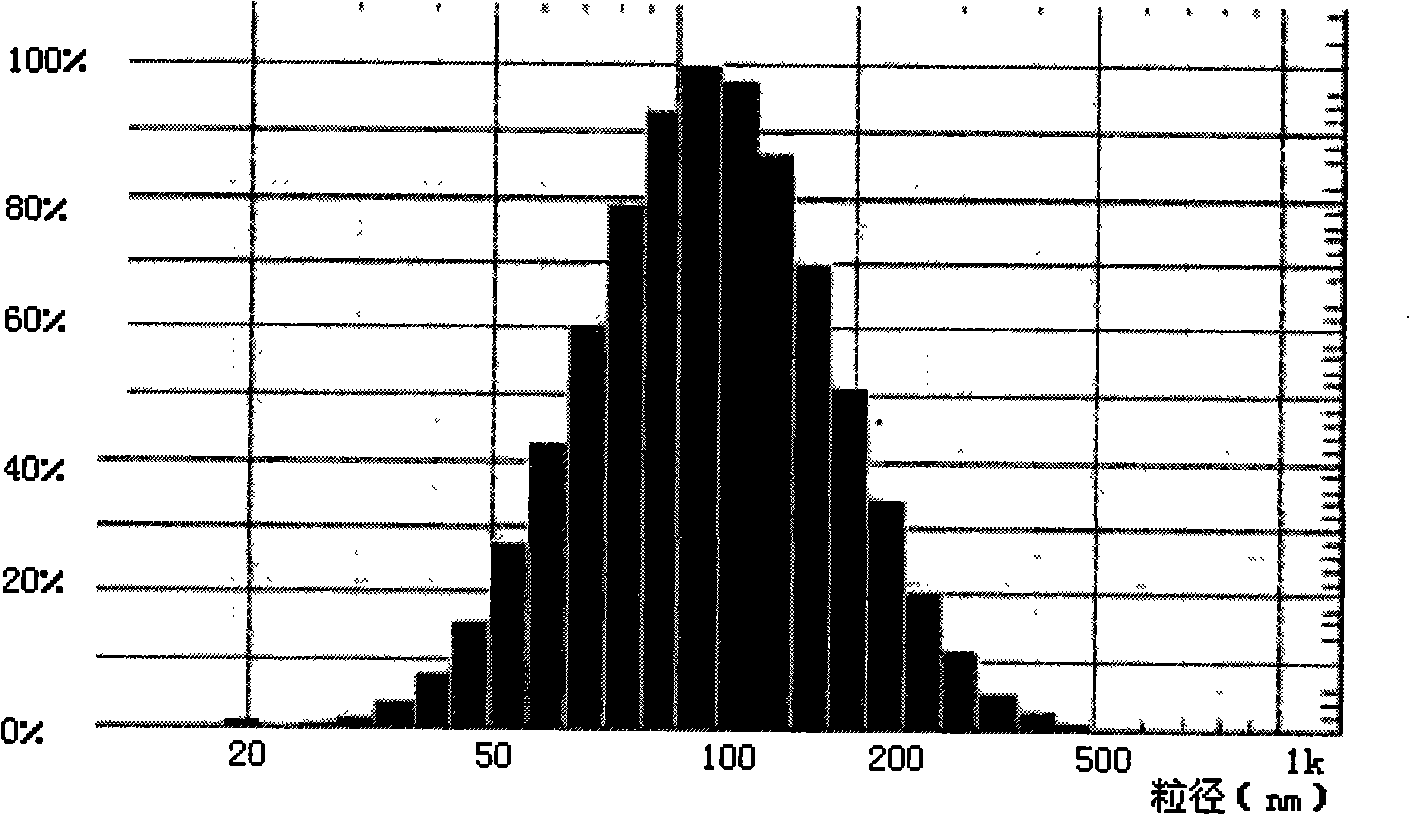
[0035]Accurately weigh 15.8mg of lecithin, 7.8mg of cholesterol, 0.6mg of PEG2000-DSPE, 0.1mg of vitamin E, and 2mg of docetaxel and dissolve them in 5ml of chloroform. After removing the chloroform and forming a uniform transparent film on the wall of the eggplant-shaped flask, add 6ml of 0.02mol / L pH6.8 phosphate buffer solution, and continue to rotate in a water bath at 37°C for 30min under normal pressure to make the film swell and hydrate. The suspension was vortexed for 10 min. After ultrasonic dispersion at room temperature for 1 min, the docetaxel stealth liposome solution was obtained, and the salt was removed by dialysis. Add 20ml of water for dilution, add 1.0g of mannitol as a proppant and freeze-dry to make freeze-dried powder injection. The average particle diameter of the sample was measured using deionized water as the dispersion medium, and the measured particle diameter was 20-500 nm, with an average particle diameter of 120 nm. see results figure 1 .
[...
Embodiment 2
[0039] Accurately weigh 20 mg of lecithin, 5.6 mg of cholesterol, 1.0 mg of PEG4000-DSPE, and 5 mg of docetaxel and dissolve them in 10 ml of dichloromethane. The resulting solution is placed in a 50 ml eggplant-shaped flask, and the dichloromethane is removed under reduced pressure in a water bath at 37°C. After forming a uniform transparent film on the wall of the eggplant-shaped flask, add 10ml of 0.01mol / L pH6.8 phosphate buffer solution, and continue to rotate in a water bath at 37°C under normal pressure for 30min to make the film swell and hydrate, and the resulting suspension Vortex for 10 min. After ultrasonic dispersion at room temperature for 1 min, the docetaxel stealth liposome solution was obtained, and the salt was removed by dialysis. Add 20ml of water for dilution, add 0.5g of mannitol and 0.4g of glucose as proppants, freeze-dry to make freeze-dried powder injection. The average particle diameter of the sample was measured using deionized water as the disper...
Embodiment 3
[0043] Get docetaxel 10mg, be dissolved in 25ml dehydrated alcohol, inject 30mg containing polyethylene glycolated polyhexadecyl cyanoacrylate (PEG6000-PHDCA, its preparation method is the conventional method in the prior art, specifically as follows : Dissolve PHDCA20mg, N,N-dicyclohexylcarboimide 10mg, N-hydroxysuccinimide 10mg in dimethyl sulfoxide 25ml, stir for 3 hours, filter to get the filtrate, PEG600020mg is dissolved in dichloromethane 15ml Add it, continue to stir for 4 hours, filter to take the filtrate, place it at 0°C for 24 hours to recrystallize, filter, freeze-dry, and obtain. After that, W / O colostrum is obtained. Then add this colostrum into 50ml, 4% PVA solution, under ice bath, then pulse ultrasonic with ultrasonic cell pulverizer for 5 minutes, obtain W / O / W double emulsion. The obtained double emulsion was added to 120 mL of a solution containing 1 wt% PVA, evaporated under reduced pressure at room temperature, and dichloromethane was removed to obtain a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com