Dual cure composition
A composition, heat curing technology, applied in the direction of coatings, polyurea/polyurethane coatings, etc., can solve the problem of unsatisfactory obtaining hardness, solvent resistance, acid resistance, scratch resistance and scratch resistance, properties Poor, insufficient hardness, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 100 grams of ditrimethylolpropane, 137 grams of acrylic acid, 0.37 grams of methoxyphenol, 2.3 grams of methanesulfonic acid and 200 grams of toluene were added to a reaction vessel equipped with a stirrer, thermometer, oxygen inlet and an azeotropic distillation column. The mixture was heated to reflux (≈110° C.) and stirred until 10 ml of water were collected. Excess acrylic acid was neutralized with 10% aqueous NaOH after cooling the reaction mixture to room temperature. The organics were separated and washed twice with water. Toluene was removed under vacuum (30 mm Hg).
[0030] The obtained product has the following characteristics:
[0031] Hydroxyl value, mg KOH / g 331
[0032] Double bond content, mmoles / g 5.3
[0033] Chroma, APHA 50
Embodiment 2
[0036] 250 g of hydroxyl-functionalized dendritic polyester (Boltorn H20, Perstorp Specialty Chemicals AB), 200 g of acrylic acid, 0.5 g of methoxyphenol, 3.5 g of methanesulfonic acid and 400 g of toluene were added to a reaction vessel equipped with a stirrer, thermometer, oxygen inlet and azeotrope. The mixture was heated to reflux (≈110° C.) and stirred until 30 ml of water were collected. Excess acrylic acid was neutralized with 10% aqueous NaOH after cooling the reaction mixture to room temperature. The organics were separated and washed twice with water. Toluene was removed under vacuum (30 mm Hg).
[0037] The obtained product has the following characteristics:
[0038] Hydroxyl value, mg KOH / g 99
[0039] Double bond content, mmoles / g 4.4
[0040] Chroma, APHA 210
Embodiment 3
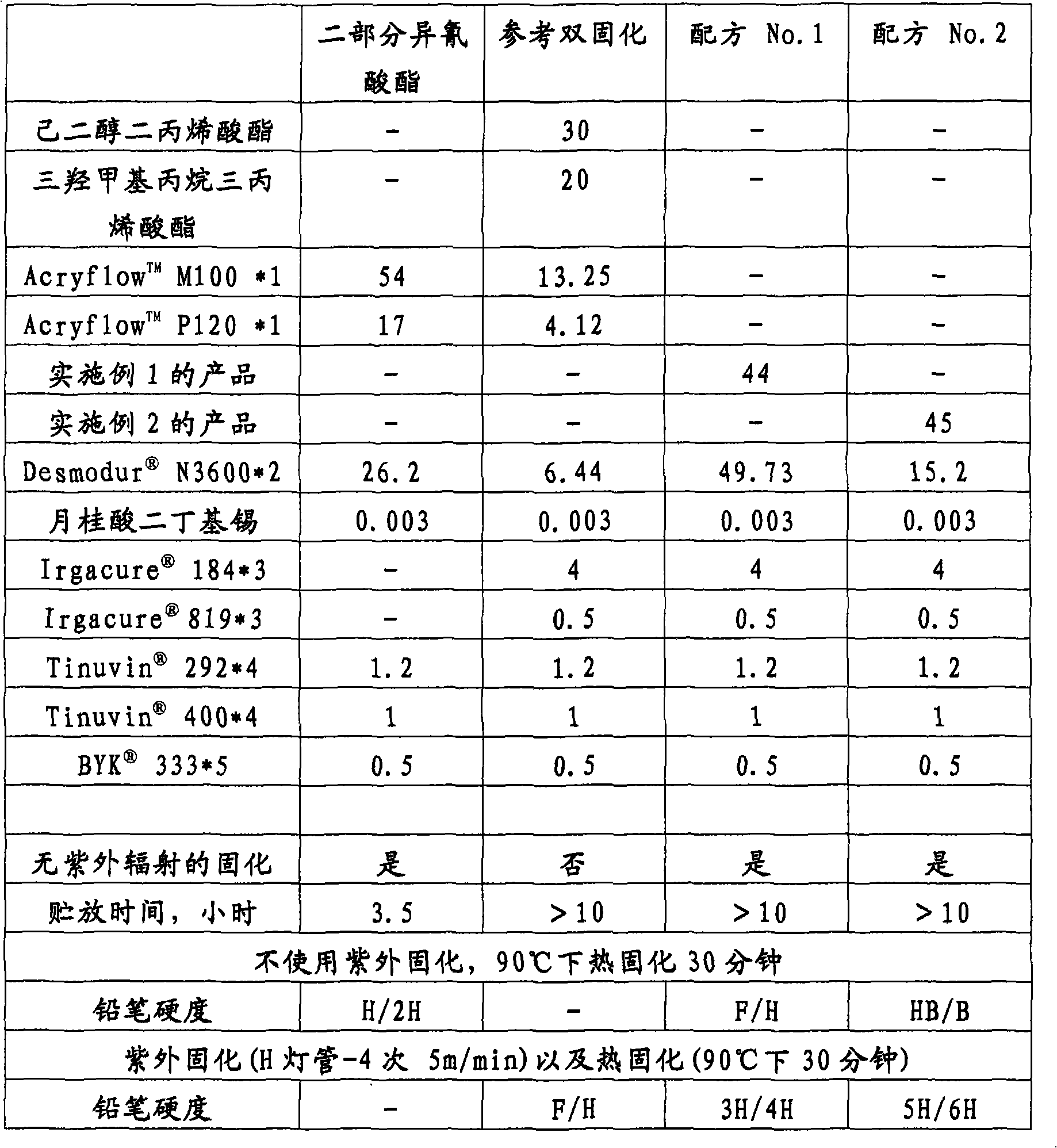
[0043] A varnish was prepared according to an embodiment of the invention (Formulations 1 and 2) and contained the product obtained in Examples 1 and 2 (component a). Two-part isocyanate-cured coatings and dual-cure coatings were prepared by ref. Coatings were made at 40°C and were applied to glass plates with a dry film thickness of ≈40 μm. The coating is cured either thermally in an oven, or thermally and UV cured. The formulation by weight and the performance of the product are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com