Preparation of bactericide propiconazole
A technology of propiconazole and fungicide, applied in the field of preparation of propiconazole, can solve problems such as air pollution, difficulty in purification and separation, and loss, and achieve the effects of avoiding recovery loss, high total yield, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
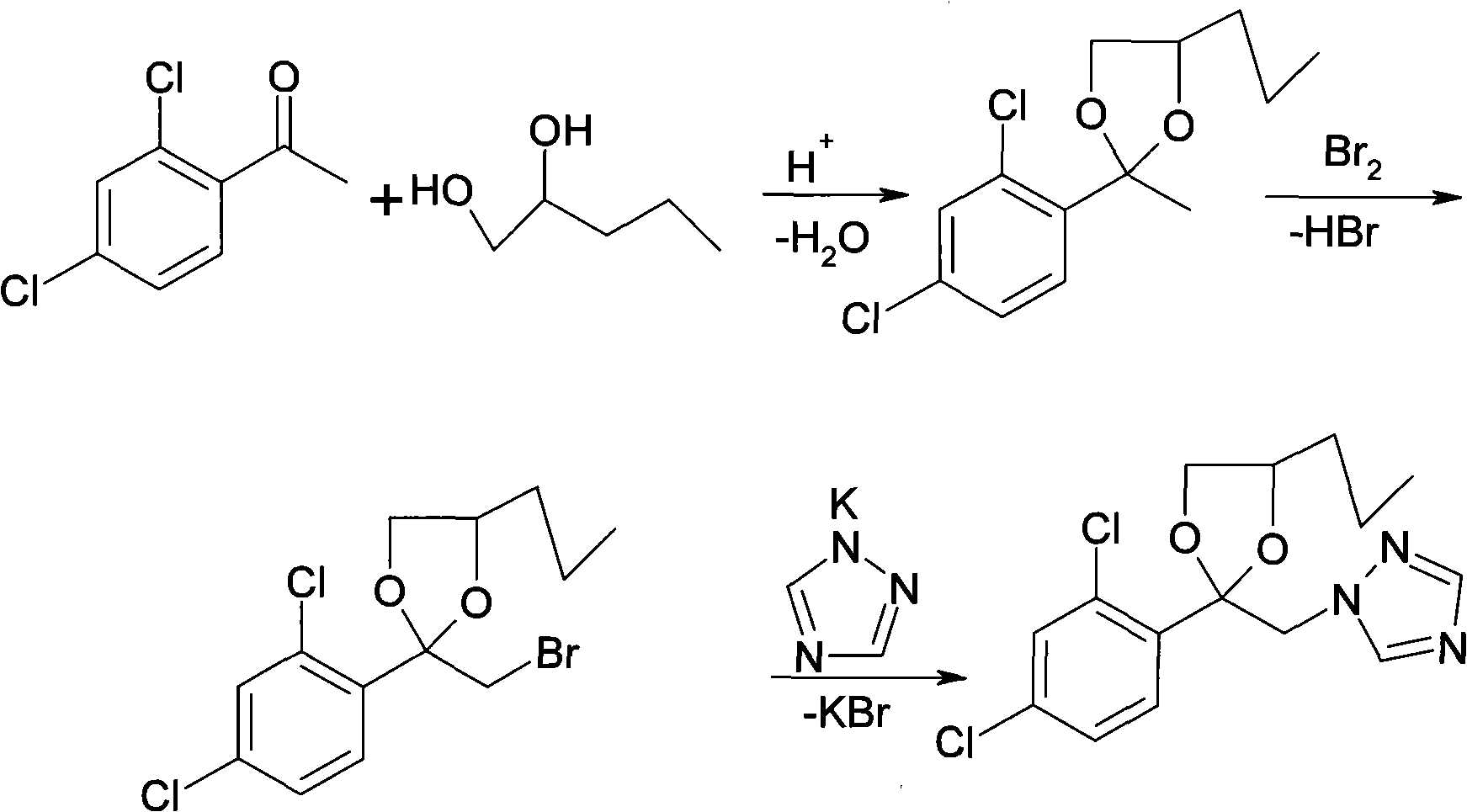
[0037] (1) Preparation of ketal
[0038] 450g (99%, 2.36mol) 2,4-dichloroacetophenone, 22.5g p-toluenesulfonic acid, 262g (98%, 2.47mol) 1,2-pentanediol and 900g hexamethylene, drop into respectively In the 2L reaction bottle of the water tank and the condenser, stir and heat up to solvent reflux, and dehydrate at normal pressure for 10 hours. GC analysis shows that the content of 2,4-dichloroacetophenone is less than 0.5%, and the reaction is completed and cooled to below 40°C. The obtained The ketal was directly used in the next step reaction.
[0039] (2) Preparation of bromoketal
[0040] To the above reaction solution, slowly add 390g (99%, 2.41mol) of liquid bromine dropwise, the dropping time is controlled within 1 to 3 hours, and the temperature is controlled at 25 to 30°C. The hydrogen bromide tail gas released during the reaction is absorbed by water . After the dropwise addition was completed, stirring was continued for 1 hour to end. Neutralize the residual aci...
Embodiment 2
[0046] (1) Preparation of ketal
[0047] 450g (99%, 2.36mol) 2,4-dichloroacetophenone, 22.5g p-toluenesulfonic acid, 262g (98%, 2.47mol) 1,2-pentanediol and 900g benzene, drop into water trap respectively In a 2L reaction flask with a condenser, stir and heat up to solvent reflux, and dehydrate at normal pressure for 10 hours. GC analysis shows that the content of 2,4-dichloroacetophenone is less than 0.5%. After the reaction is completed, it is cooled to below 40°C. The ketone was directly used in the next reaction.
[0048] (2) Preparation of bromoketal
[0049] To the above reaction solution, slowly add 390g (99%, 2.41mol) of liquid bromine dropwise, the dropping time is controlled within 1 to 3 hours, and the temperature is controlled at 25 to 30°C. The hydrogen bromide tail gas released during the reaction is absorbed by water . After the dropwise addition was completed, stirring was continued for 1 hour to end. Neutralize the residual acid in the system with 10% aque...
Embodiment 3
[0055] (1) Preparation of ketal
[0056] 450g (99%, 2.36mol) 2,4-dichloroacetophenone, 22.5g p-toluenesulfonic acid, 262g (98%, 2.47mol) 1,2-pentanediol and 900g hexamethylene, drop into respectively In the 2L reaction bottle of the water tank and the condenser, stir and heat up to solvent reflux, and dehydrate at normal pressure for 10 hours. GC analysis shows that the content of 2,4-dichloroacetophenone is less than 0.5%, and the reaction is completed and cooled to below 40°C. The obtained The ketal was directly used in the next step reaction.
[0057] (2) Preparation of bromoketal
[0058] To the above reaction solution, slowly add 390g (99%, 2.41mol) of liquid bromine dropwise, the dropping time is controlled within 1 to 3 hours, and the temperature is controlled at 25 to 30°C. The hydrogen bromide tail gas released during the reaction is absorbed by water . After the dropwise addition was completed, stirring was continued for 1 hour to end. Neutralize the residual aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com