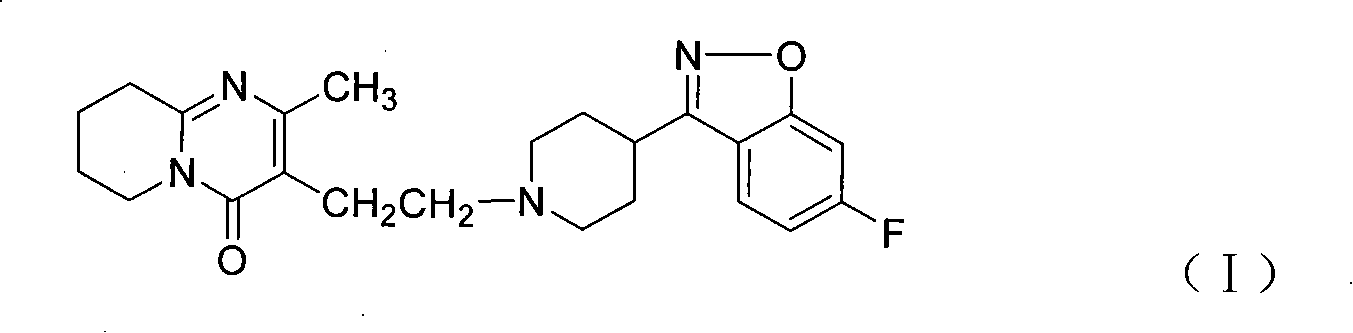
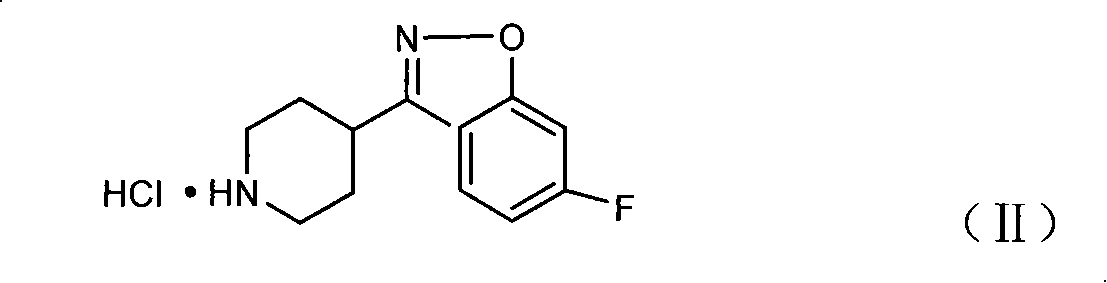
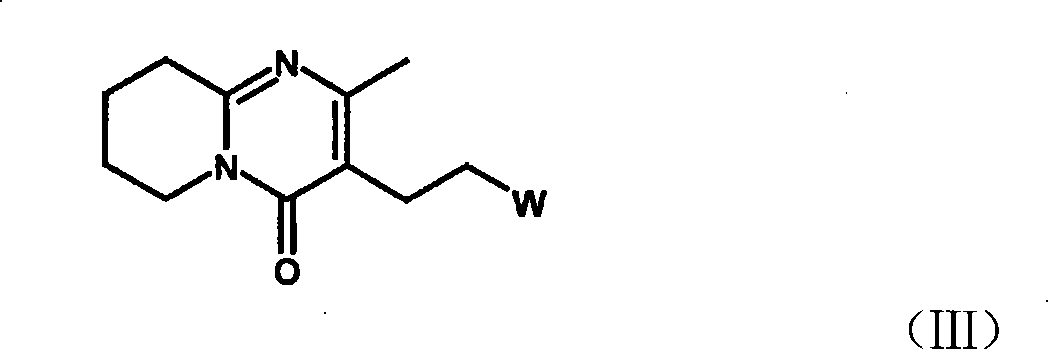
Method for preparing 6-fluoro-3-(4- piperidyl)-1,2-benzo isoxazole hydrochlorate
A technology of benzisoxazole hydrochloride and piperidinyl, applied in the field of chemical synthesis, can solve problems such as low water solubility, difficult separation, and affecting the yield of risperidone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Under stirring at room temperature, mix 13.6 g (0.049 mol) of 2,4-difluorophenyl (4-piperidinyl) ketone oxime hydrochloride, 6.9 g (0.123 mol) of solid KOH powder and 109 ml of acetone, and heat up to 55 ~60°C, reflux reaction for 2 hours; after the reaction, add an appropriate amount of anhydrous sodium sulfate to dry, cool to room temperature, stir for 30 minutes, and filter; pass HCl gas into the filtrate, and crystallize; filter and dry to obtain 6-fluoro - 3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride 12.25g, yield 97.47%, content>99%.
Embodiment 2
[0029] Under stirring at room temperature, 13.6 g (0.049 mol) of 2,4-difluorophenyl (4-piperidinyl) ketone oxime hydrochloride, 8.23 g (0.147 mol) of solid KOH powder and N, N-dimethyl Mix 136ml of formamide, heat up to 40-45°C, and reflux for 3 hours; after the reaction, add an appropriate amount of anhydrous sodium sulfate, cool down to room temperature, stir for 30 minutes, and filter; pass HCl gas into the filtrate, and crystallize; filter , and dried to obtain 11.69 g of 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride, with a yield of 93.01% and a content of >99%.
Embodiment 3
[0031] Under stirring at room temperature, mix 24 g (0.1 mol) of 2,4-difluorophenyl (4-piperidinyl) ketone oxime, 11.2 g (0.2 mol) of solid KOH powder and 120 ml of N, N-dimethylacetamide , heated up to 45-50°C, and refluxed for 1 hour; after the reaction, add an appropriate amount of anhydrous sodium sulfate, cool down to room temperature, stir for 30 minutes, and filter; pass HCl gas into the filtrate, and crystallize; filter, dry, and get 24.55 g of 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride, yield 95.7%, content>99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com