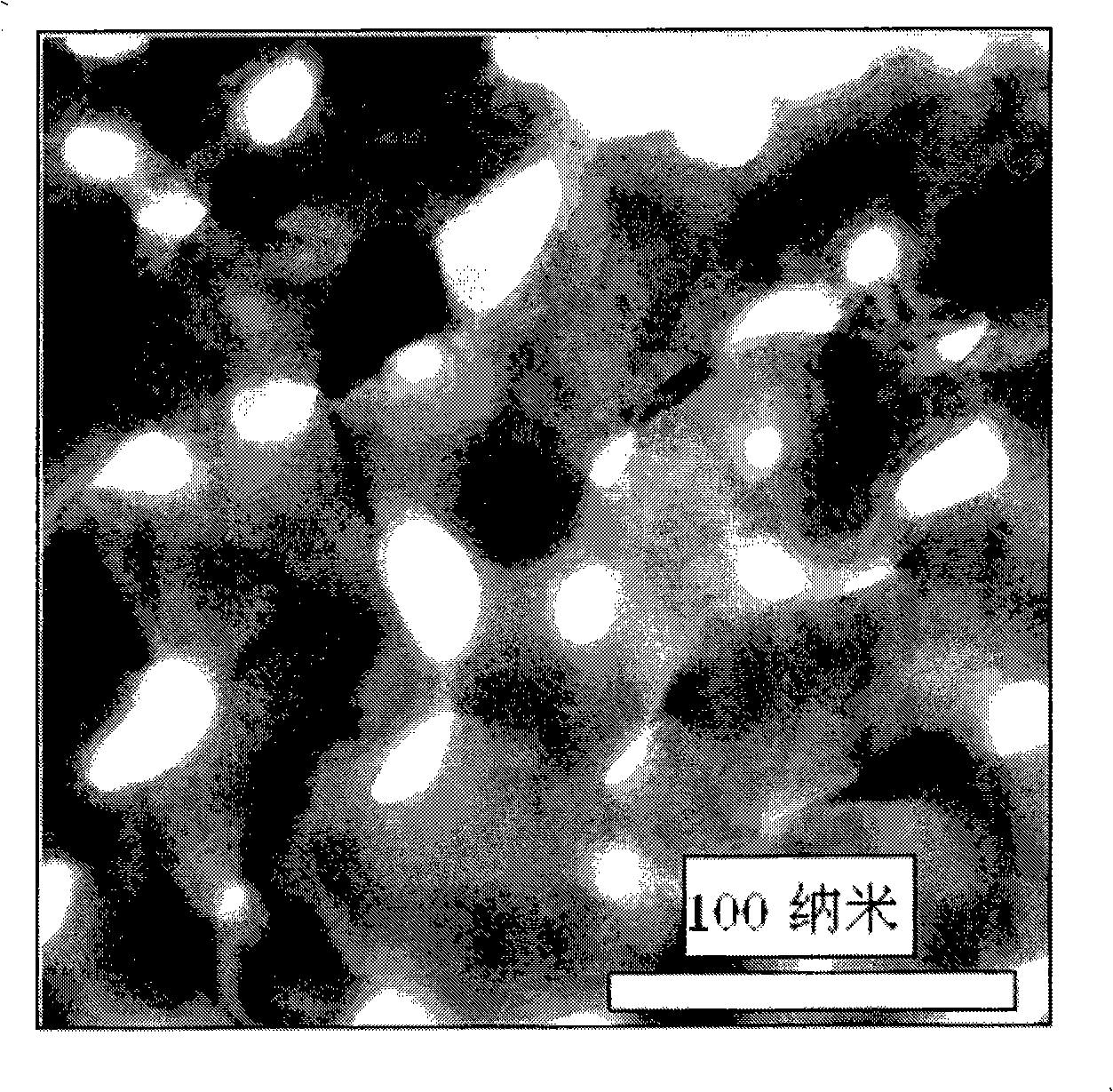
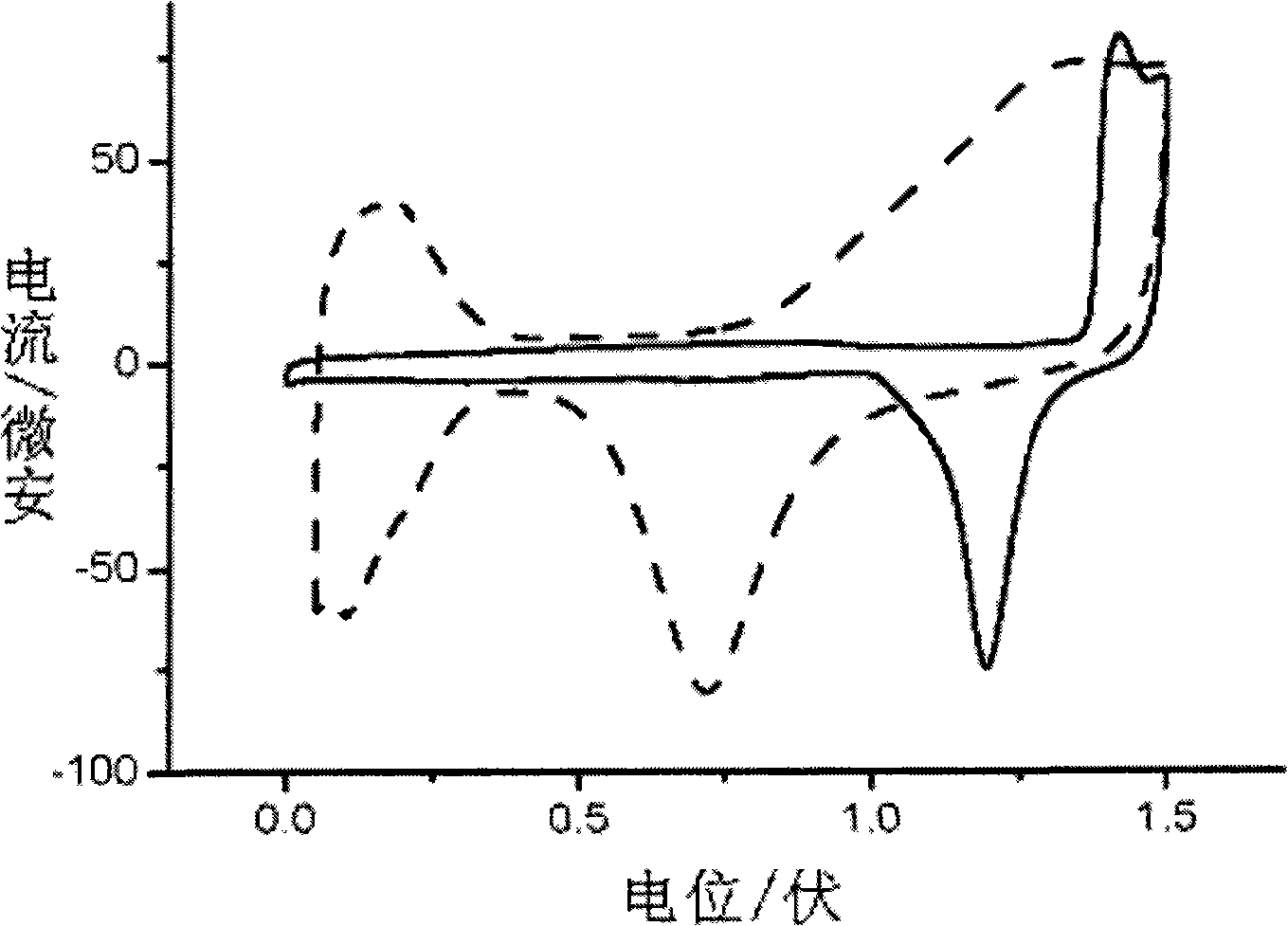
Nano porous gold-loaded ultrathin platinum metallic film catalyst and preparation method thereof
A nanoporous gold and platinum-series metal technology is applied in the field of nanoporous gold-supported ultra-thin platinum-series metal film catalysts and their preparation, and the effect of reducing the load is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) A gold-silver alloy foil with a thickness of 25 microns, a width of 1 cm, a length of 2 cm, and a gold atomic percentage of 26 at.% is placed in nitric acid with a concentration of 65-68 wt.%, and the corrosion time is 60 minutes. Then use deionized water to clean the nitric acid remaining in the pores to obtain nanoporous gold;
[0038] (2) At 25°C, a three-electrode electrochemical system was adopted, the nanoporous gold prepared above was used as the working electrode, the platinum sheet was used as the counter electrode, and the mercury-mercurous sulfate electrode was used as the reference electrode. 2 SO 4 +0.5mM CuSO 4 , deposited at -0.45V potential for 2 minutes, then transferred to 1mM PdCl 2 Replacement in the medium for 10 minutes to obtain a composite metal catalyst with a monoatomic layer of palladium film covering the porous gold surface.
Embodiment 2
[0040] (1) A gold-silver alloy foil with a thickness of 0.1 micron, a width of 2 cm, a length of 2 cm, and a gold atomic percentage of 26 at.% is placed in nitric acid with a concentration of 65-68 wt.%, and the corrosion time is 90 minutes. Then use deionized water to clean the nitric acid remaining in the pores to obtain nanoporous gold;
[0041] (2) At 25°C, a three-electrode electrochemical system was adopted, with the nanoporous gold prepared above as the working electrode, the platinum sheet as the counter electrode, and the mercurous sulfate electrode as the reference electrode, 0.5M H 2 SO 4 +0.5mM CuSO 4 , deposited at -0.45V potential for 5 minutes, then transferred to 1mM K 2 PtCl 6 Replacement in 10 minutes. Afterwards, it was washed with deionized water to obtain a composite metal catalyst in which half of the atomic layer of platinum covered the surface of the porous gold.
Embodiment 3
[0043] (1) A gold-silver alloy foil with a thickness of 0.1 micron, a width of 1 cm, a length of 1 cm, and a composition of 26 at.% gold atomic percentage is placed in nitric acid with a concentration of 65-68 wt.%, and the corrosion time is 60 minutes. Then use deionized water to clean the nitric acid remaining in the pores to obtain nanoporous gold;
[0044] (2) At 25°C, using a three-electrode electrochemical system, with the nanoporous gold prepared above as the working electrode, the platinum sheet as the counter electrode, and the mercurous sulfate electrode as the reference electrode, in 0.5M H 2 SO 4 +0.5mM CuSO 4 solution, deposited at a potential of -0.45V for 2 min, then transferred to 1 mM K 2 PtCl 4 Replacement in 10 minutes; then in the above-mentioned containing CuSO 4 In dilute sulfuric acid solution, deposit copper at -0.4V potential for 2 minutes, and in 1mM K 2 PtCl 4 The displacement was carried out in the medium for 10 minutes, and the composite nano...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com