Organic photoelectric functional material based on trisubstituted triindene structure unit and uses thereof
A technology for optoelectronic functional materials and structural units, applied in the fields of luminescent materials, photovoltaic power generation, organic chemistry, etc., can solve problems such as limiting the scope of application, reduce quenching, improve amorphous properties and film-forming properties, and inhibit crystallinity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Oligofluorene monoboronate monomers such as intermediates 3, 4, 5 and 6 were prepared by the following method. After carrying out alkylation treatment to the three indene nucleus to increase its solubility, the catalytic amount I 2 Under the effect of and sufficient amount of Br 2 The reaction can produce the important hexabromotriindene monomer Br 6 Tr. Then in Pd(PPh 3 ) 4 Under the action of catalyst, Br 6 Tr was subjected to multiple coupling Suzuki reactions with 3, 4, 5 or 6, etc., and separated by column chromatography to obtain a six-arm structure oligomeric alkylfluorene-modified triindenes derivative material Trn (Tr1:n= 1; Tr2:n=2; Tr3:n=3; Tr4:n=4, . . . ).
[0079]
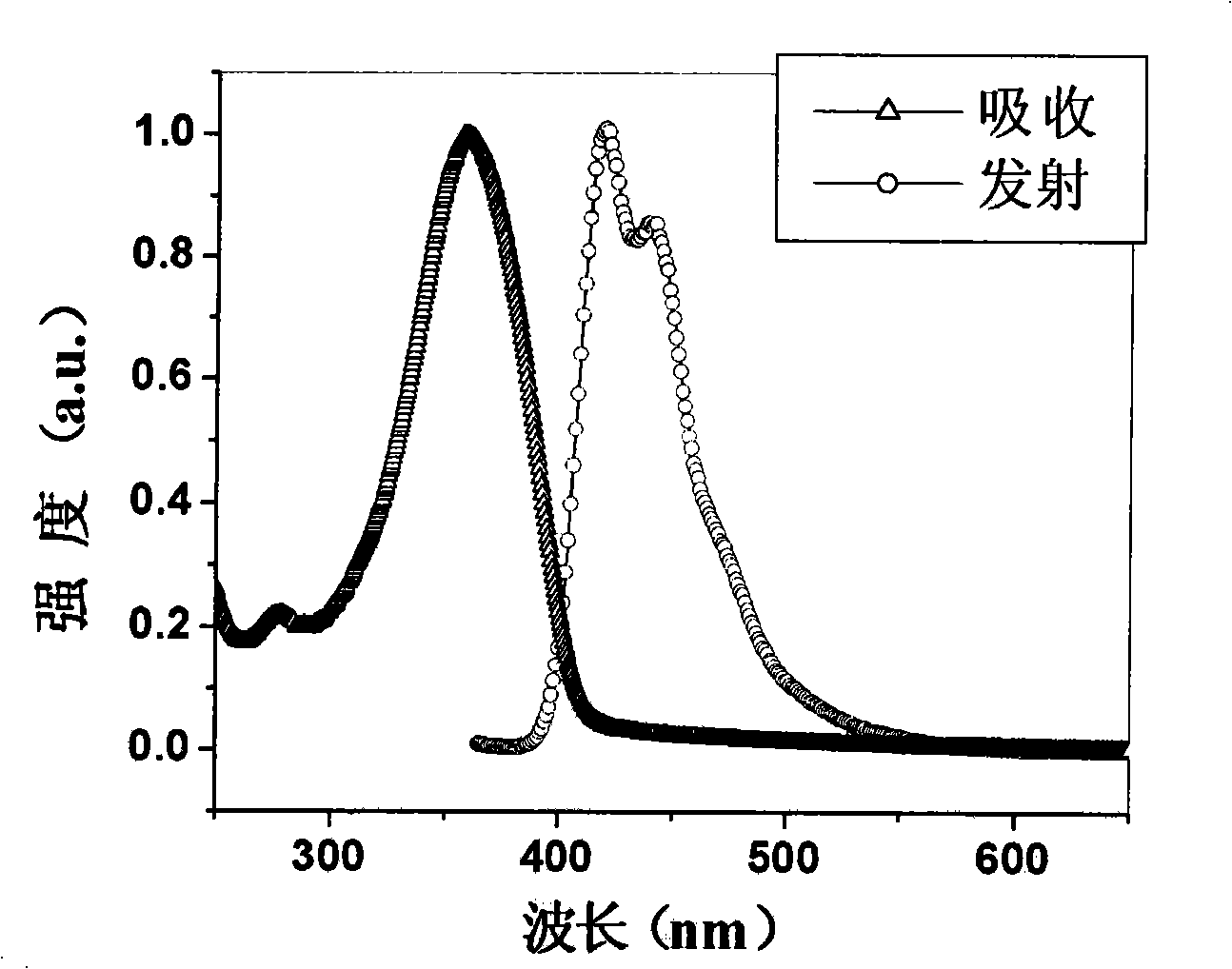
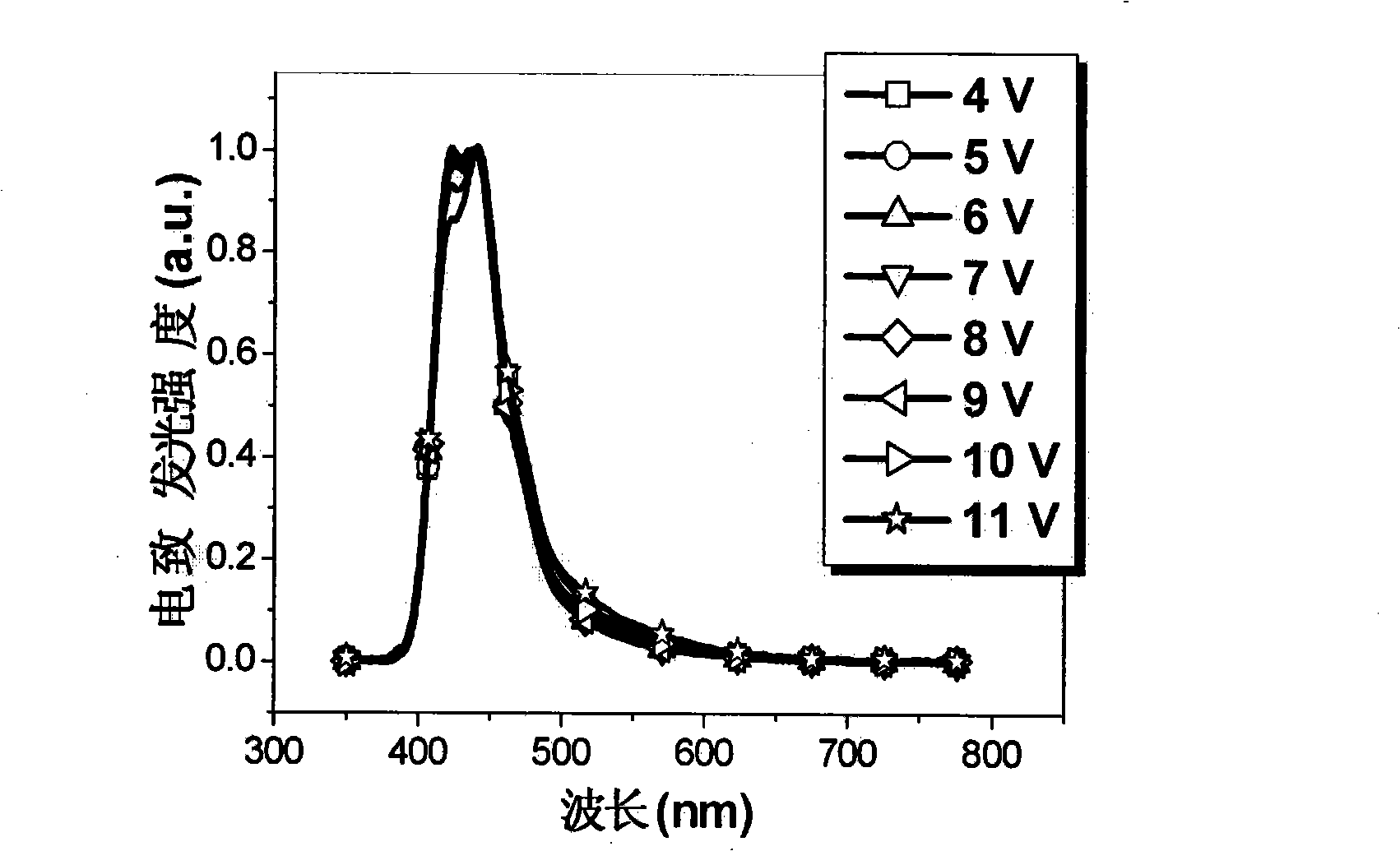
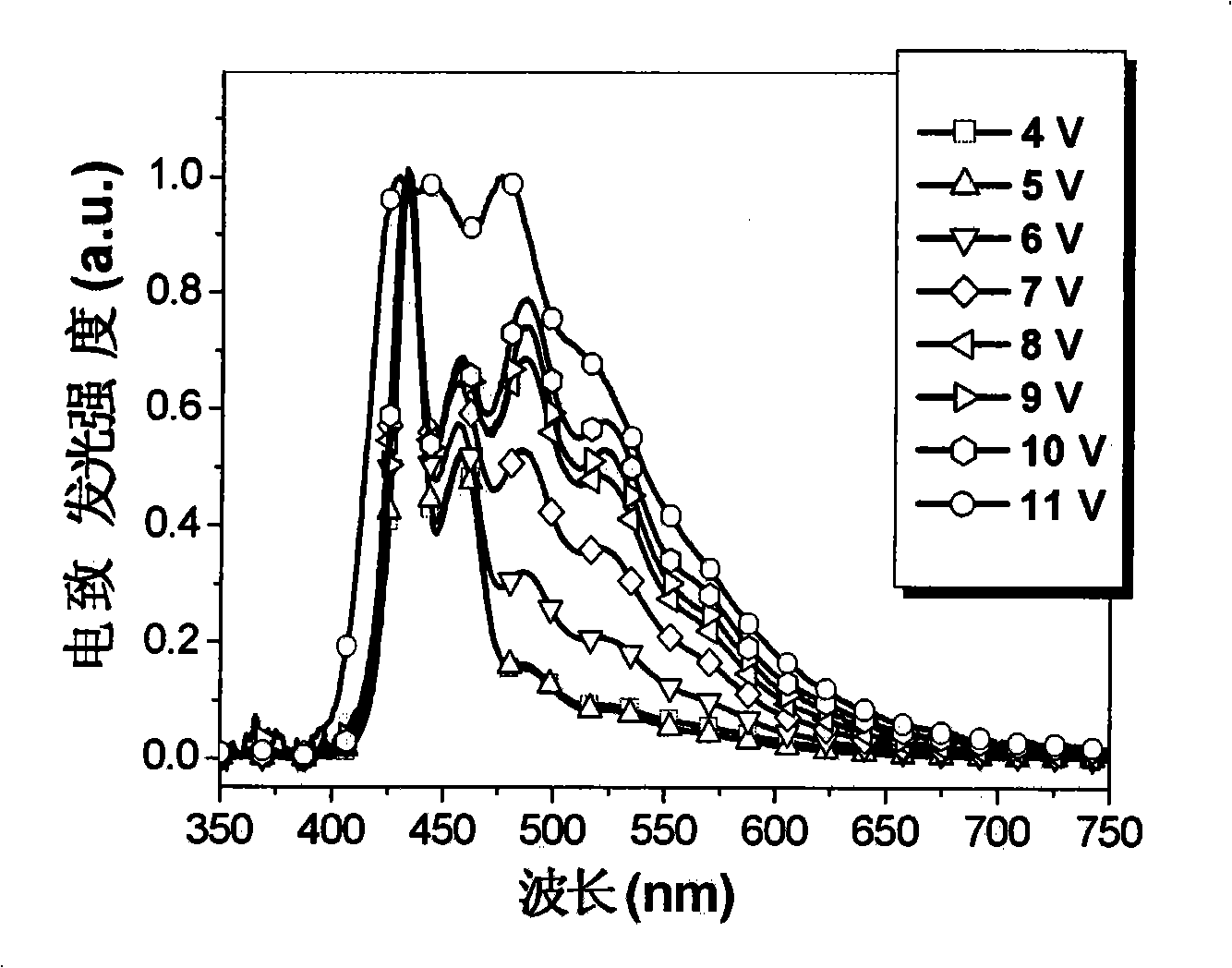
[0080] attached figure 1 Shown is the absorption spectrum and fluorescence emission spectrum of Tr3 in the thin film state. Its maximum absorption peak appears at 359nm, and its maximum emission peak appears at 420nm, emitting pure and bright dark blue light. Electroluminescence test...
Embodiment 2
[0082] With embodiment 1, prepare intermediate 1 and Br 6 Tr, prepared by the following method to obtain intermediates 7, 8 and 9, etc., and then in Pd (PPh 3 ) 4 Under the action of catalyst, Br 6 Tr was subjected to multiple coupling Suzuki reactions with 7, 8 or 9, etc., and separated by column chromatography to obtain a six-arm structure oligomeric alkylfluorenetriindenene derivative material TrCzn (TrCz1: n=1; TrCz2: n=2; TrCz3: n=3; …). Compared with the Trn series materials, the introduction of the carbazole unit effectively increases the HOMO energy level of the TrCzn series target materials, improves the hole injection and transport properties of the target materials, and maintains the six-arm structure of the oligomeric alkyl fluorene three Excellent light emission properties of indene derivative materials.
[0083]
Embodiment 3
[0085] With embodiment 1, prepare intermediate 1 and Br 6 Tr, prepare intermediates 10, 11, 12 and 13 etc. with the following method, then in Pd (PPh 3 ) 4 Under the action of catalyst, Br 6 Tr was reacted with 11, 12 or 13 by multiple coupling Suzuki reaction respectively, and separated by column chromatography to obtain a six-arm structure oligomeric alkyl fluorene tri-indenes derivative material TrPhn (TrPh1: n=1; TrPh2: n=2; TrPh3: n=3; …). Compared with Trn series materials, the introduction of alkylphenyl capping groups enhances the solubility and solid-state film-forming properties of the target material, making this material suitable for preparing high-quality amorphous films by simple solution methods such as spin coating .
[0086]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com