Method for preparing sodium chloride and magnesium sulphate heptahydrate with brine
A technology of magnesium sulfate heptahydrate and sodium chloride, which is applied in the field of salt production, can solve the problems of large loss of magnesium sulfate, and achieve the effect of simple equipment and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
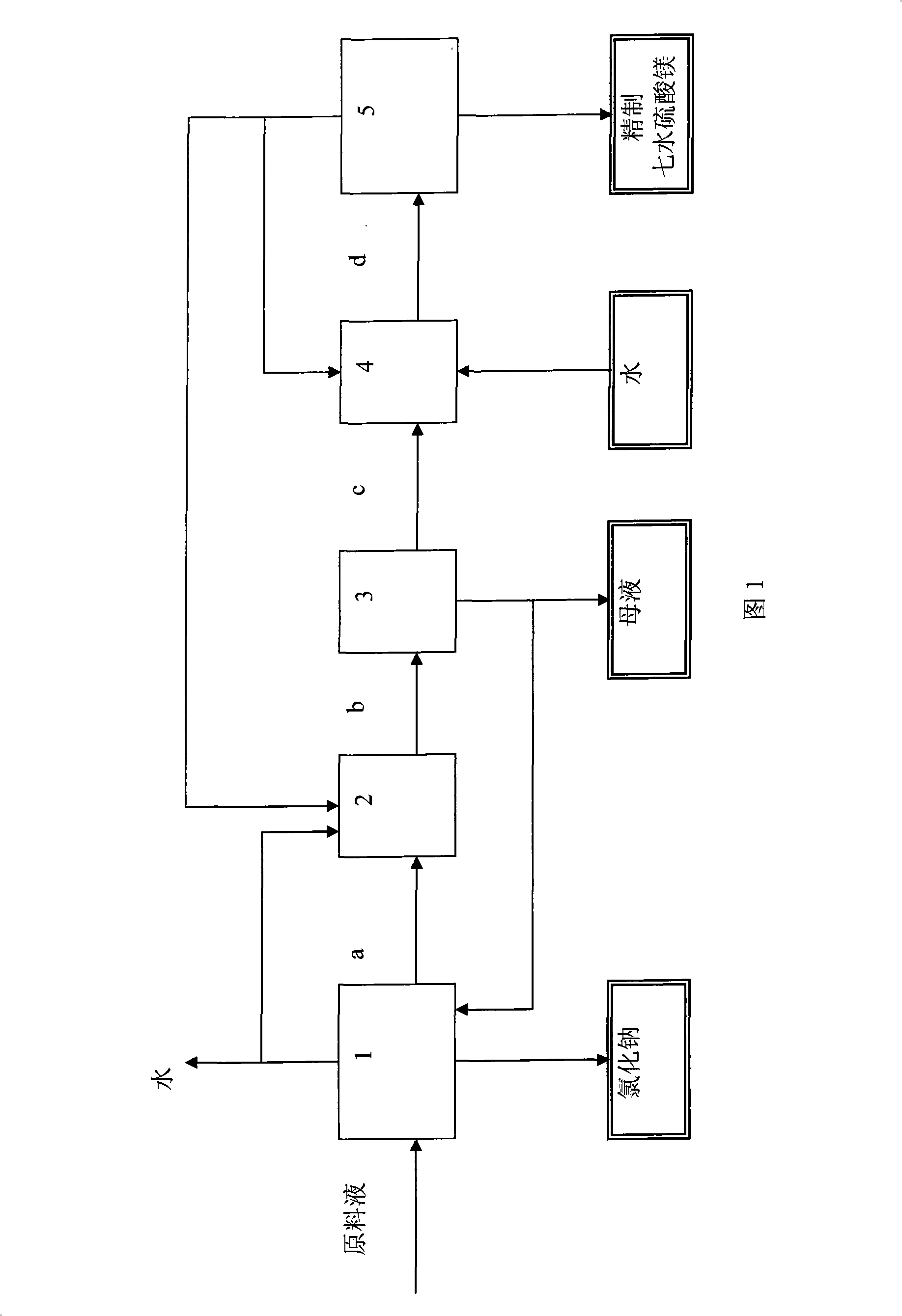
[0025] Take 1000ml potassium-containing brine, the composition concentration KCl: 15.0g / l, MgCl 2 : 81.0g / l, MgSO 4 : 93.0g / l, NaCl: 183.0g / l, H 2 O: 890.0g / l, of which SO 4 2- :Na + Molar ratio = 0.25, SO 4 2- : Mg 2+ Molar ratio of 0.48, heating with an electric heating mantle in a three-necked flask, connecting the three-necked flask to a vacuum pump, and controlling the pressure to make the feed liquid boil and evaporate at 55°C, and evaporate 395g of water. At this time, the liquid phase SO 4 2- :Na + The molar ratio is about 1.8, solid-liquid separation is carried out, the solid phase is washed with a saturated aqueous sodium chloride solution, and after drying, 153g of sodium chloride is obtained with a purity of 99.5%. Add 80g of water to the salt-making mother liquor, pre-cool it to 30°C, and add it to the crystallized In the liquid crystallizer (the crystallization liquid is a saturated solution of magnesium sulfate), the temperature of the crystallizer is -...
Embodiment 2
[0027] Take 1000ml of intercrystalline brine from a certain salt lake, the concentration of which is MgCl 2 : 120.0g / l, MgSO 4 : 118.0g / l, NaCl: 140g / l, H 2 O: 818.0g / l, of which SO 4 2- :Na + Molar ratio = 0.41, SO 4 2- :Mg 2+Molar ratio=0.44, heated with an electric heating mantle in a three-necked flask, the three-necked flask was connected to a vacuum pump, and the feed liquid was boiled and evaporated at 60°C by controlling the pressure, and 218.6g of water was evaporated, and the SO in the liquid phase 4 2- :Na + The mol ratio is 1.77, carry out solid-liquid separation, solid phase is washed with saturated sodium chloride aqueous solution, obtains 55g sodium chloride after drying, and purity is at 99.5%; In the crystallizer of the crystallization solution (the crystallization solution is a saturated solution of magnesium sulfate), after fully crystallizing, the solid phase is separated, and the solid phase is added to a saturated solution of magnesium sulfate at...
Embodiment 3
[0029] Take 1000g of seawater to make salt flat brine and dry saturated brine, the composition concentration is Ca 2+ : 0.038%, Mg 2+ : 2.599%, K + : 0.711%, Na + : 7.86%, Cl - : 17.2%, SO 2- 4 : 4.116%, H 2 O: 67.6%, of which SO 4 2- :Na + Molar ratio = 0.127, SO 4 2- : Mg 2+ Molar ratio = 0.40, heating with an electric heating mantle in a three-necked flask, connected to a vacuum pump, and controlling the pressure to make the feed liquid boil and evaporate at 60°C, evaporate 380.g of water, and the SO in the liquid phase 4 2- :Na + Molar ratio 2.05, carry out solid-liquid separation, solid phase is washed with saturated sodium chloride aqueous solution, obtains 170g sodium chloride after drying, and purity is at 99.5%; In the crystallizer (the crystallization liquid is a saturated solution of magnesium sulfate), after fully crystallizing, separate the obtained solid phase, add magnesium sulfate heptahydrate recrystallization mother liquor, and make a saturated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com