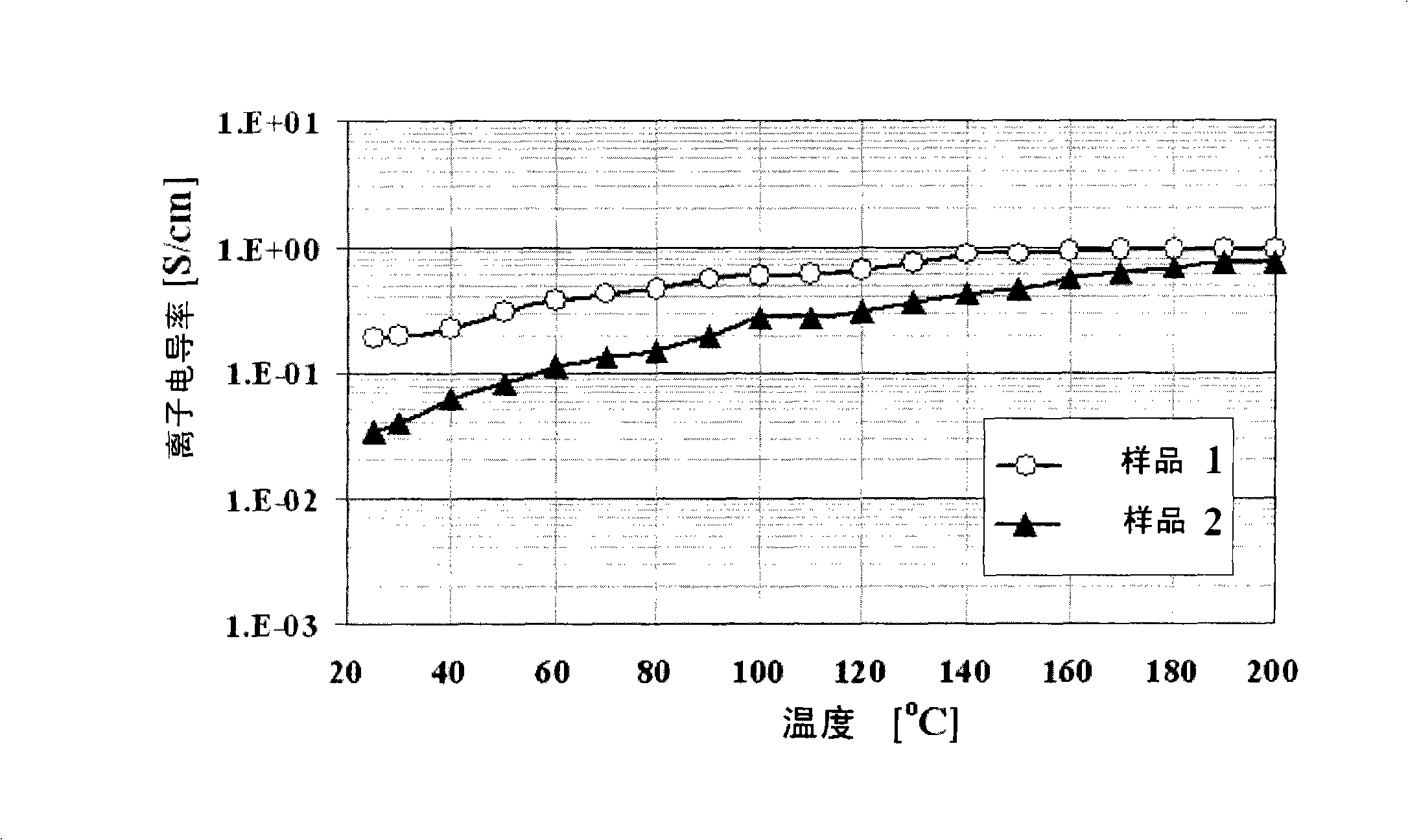
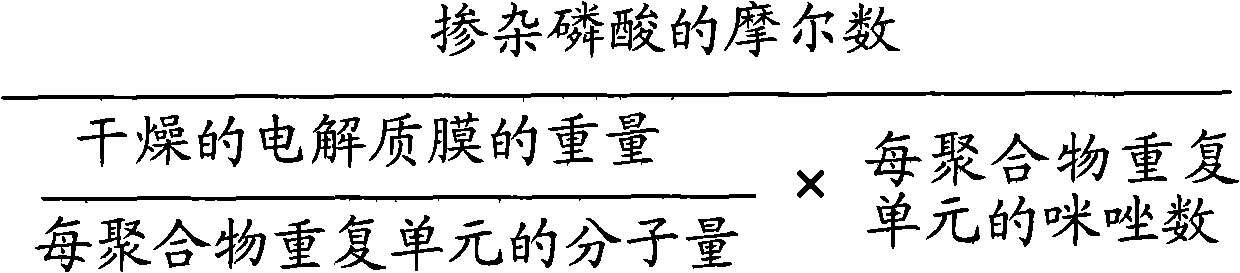
Novel metal (III) -chromium-phosphate complex and use thereof
A technology of complexes and phosphates, applied in the directions of phosphates, chromium compounds, phosphorus compounds, etc., can solve the problems of decreased ionic conductivity and failure to reach hydrogen ion conductivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: Preparation of polyparabenzimidazole copolymer
[0070] The terephthalic acid and 3,3',4,4'-tetraaminobiphenyl (3,3',4,4'-tetraaminobiphenyl) used for the polymerization reaction were dried in vacuum at 80°C for at least 24 hours. In addition, using the polyphosphoric acid provided by JUNSEI (P 2 O 5 : 85%, H 3 PO 4 : 115%) as a solvent.
[0071] Under a nitrogen atmosphere, 80 g of polyphosphoric acid was added to a reactor equipped with a stirrer, and the temperature of the reactor was increased to 170° C. to easily stir the polyphosphoric acid. Then, 3.000 g (9.334 mmol) of 3,3′,4,4′-tetraaminobiphenyl and 2.326 g (9.334 mmol) of terephthalic acid were added to the polyphosphoric acid, and the mixture was stirred for 48 hours. Then, 80 g of polyphosphoric acid and phosphoric acid were additionally added to the solution, and stirred to reduce the viscosity of the solution. As a result, a poly(2,2-p-(phenylene)-5,5-bibenzimidazole) polyphosphoric acid solution...
Embodiment 2
[0072] Example 2: Preparation of aluminum-chromium-phosphate complex
[0073] Use Al(OH) in 85% phosphoric acid solution 3 And CrO 3 To prepare Al(OH) 3 : CrO 3 : H 3 PO 4 =3:1:9 (molar ratio) aluminum-chromium-phosphate complex. For this purpose, Al(OH) is added to the 85% phosphoric acid solution 3 And dissolve at 80°C for 20 minutes until a clear liquid is formed. Then, add CrO to it 3 And the mixture was stirred for 1 hour while slowly adding methanol to it, thereby preparing an aluminum-chromium-phosphate complex [Al 3 Cr(HPO 4 ) 3 (H 2 PO 4 ) 6 ].
Embodiment 3
[0074] Example 3: Preparation of polyp-benzimidazole / aluminum-chromium-phosphate composite material
[0075] To 100 g of the 15 wt% polyp-benzimidazole polyphosphate solution prepared in Example 1, 10 g of the aluminum-chromium-phosphate prepared in Example 2 was added. The mixture was stirred at 150°C for 6 hours, thereby preparing a solution of about 50% by weight of polyp-benzimidazole / aluminum-chromium-phosphate composite material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com