Method for preparing cinnamic alcohol by hydrogen transfer reaction of benzalacet aldehyde
A technology of cinnamaldehyde and cinnamyl alcohol is applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve problems such as unreported, and achieve the effects of low cost, simple preparation conditions, and improved conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
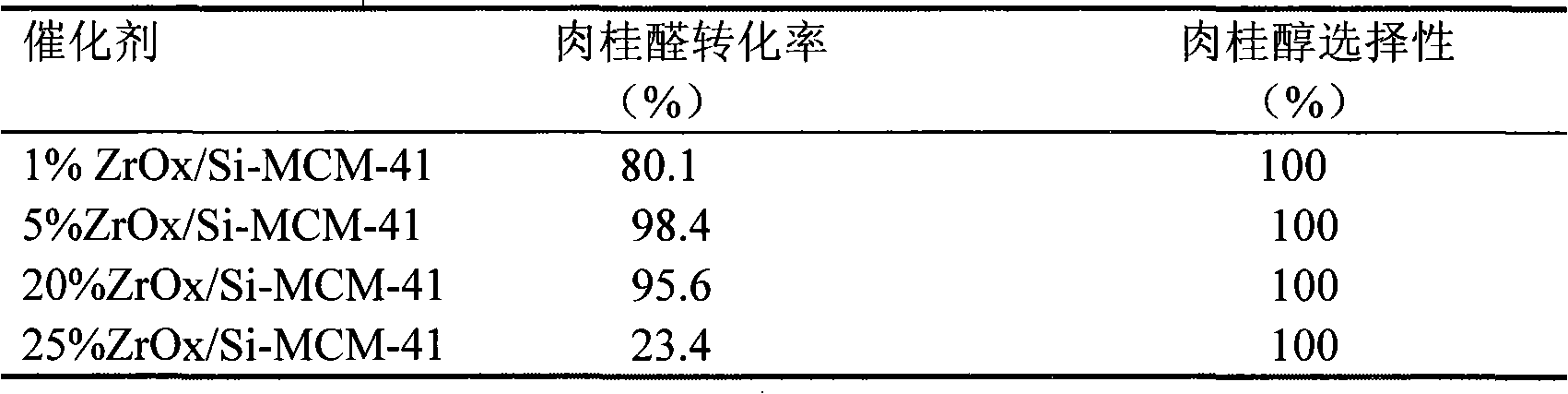
[0024] Embodiment 1. is catalyst with ZrOx / Si-MCM-41, ZrO loading is different
[0025] The preparation process of ZrOx / Si-MCM-41 catalyst is as follows: Weigh 0.112~2.8g Zr(NO 3 ) 4 ·5H 2 O, dissolved in 200ml of deionized water, added 3g of all-silicon MCM-41 mesoporous molecular sieve carrier, stirred for 24h, distilled under reduced pressure at 40-60°C for 4-6h, centrifuged, and dried at 100°C for 10h to obtain the Zr loading capacity 1% to 25% ZrOx / Si-MCM-41 catalyst.
[0026] Take 1 g of catalysts with different loads and treat them in air at 573K for 5 hours. The hydrogen transfer reaction of cinnamaldehyde was carried out in a 25ml round bottom flask under normal pressure and a reaction temperature of 76°C. Add 3mmol cinnamaldehyde, 60mmol isopropanol, 0.3g catalyst and a certain amount of triethylamine (the molar ratio of triethylamine to Zr is 1.5:1) into the flask, stir magnetically, and reflux water at 20°C to condense. Both isopropanol and cinnamaldehyde were...
Embodiment 2
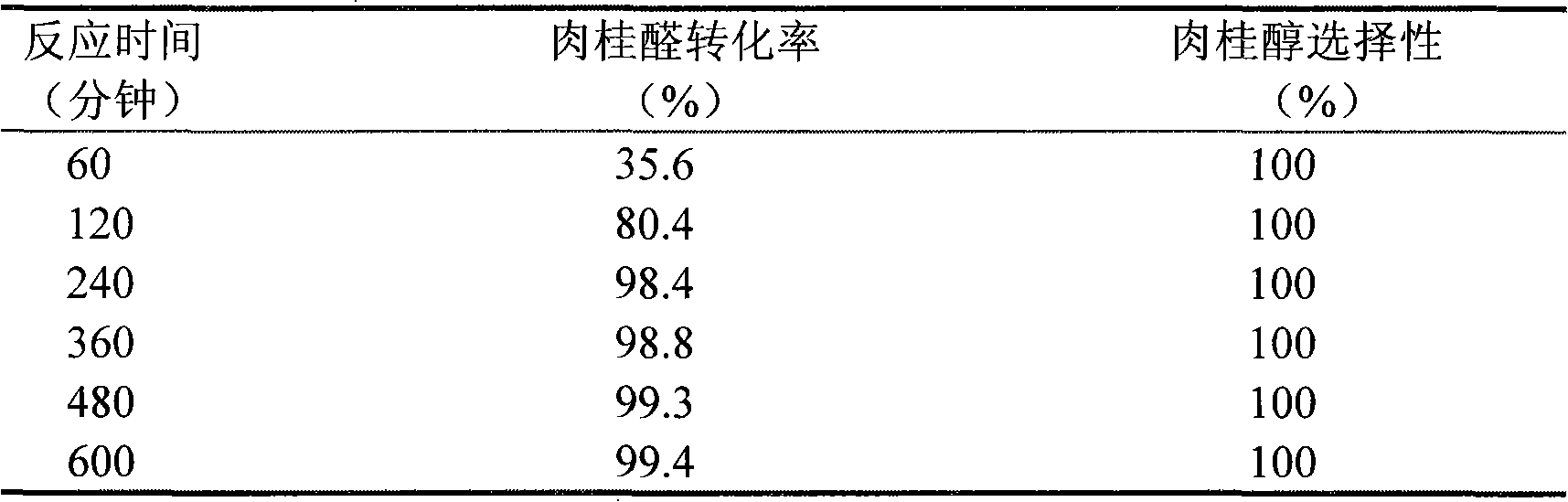
[0029] Embodiment 2. is catalyst with 5%ZrOx / Si-MCM-41, and reaction time is different
[0030] The preparation process of the 5%ZrOx / Si-MCM-41 catalyst and the pretreatment process before use are the same as those described in Example 1, and the reaction and analysis conditions are also basically the same, the difference being that regular sampling and analysis during the reaction process. Table 2 shows the conversion of cinnamaldehyde over the 5% ZrOx / Si-MCM-41 catalyst with the reaction time.
[0031] The conversion situation of cinnamaldehyde on 5%ZrOx / Si-MCM-41 catalyst under the different reaction times of table 2
[0032]
Embodiment 3
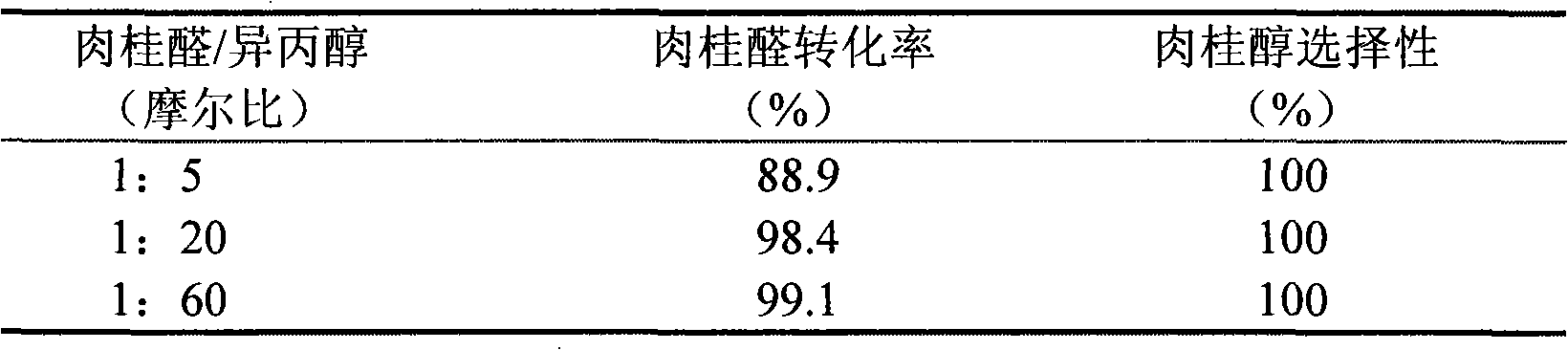
[0033] Embodiment 3. is catalyst with 5%ZrOx / Si-MCM-41, and raw material charging ratio is different
[0034] The preparation process of 5%ZrOx / Si-MCM-41 catalyst and the pretreatment process before use are identical with that described in embodiment 1, and reaction and analysis condition are also substantially identical, and difference is the feed ratio of cinnamaldehyde and Virahol different. Table 3 shows the conversion of cinnamaldehyde on 5% ZrOx / Si-MCM-41 catalyst with different feed ratios of cinnamaldehyde and isopropanol.
[0035] The conversion situation of cinnamaldehyde on 5%ZrOx / Si-MCM-41 catalyst when table 3 different cinnamaldehyde and isopropanol feed ratio
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com