Acebrophylline sugar-free type granular formulation and preparation method thereof
An acetobromophylline, sugar-free technology, applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, respiratory system diseases, etc. Easy to take and quick absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
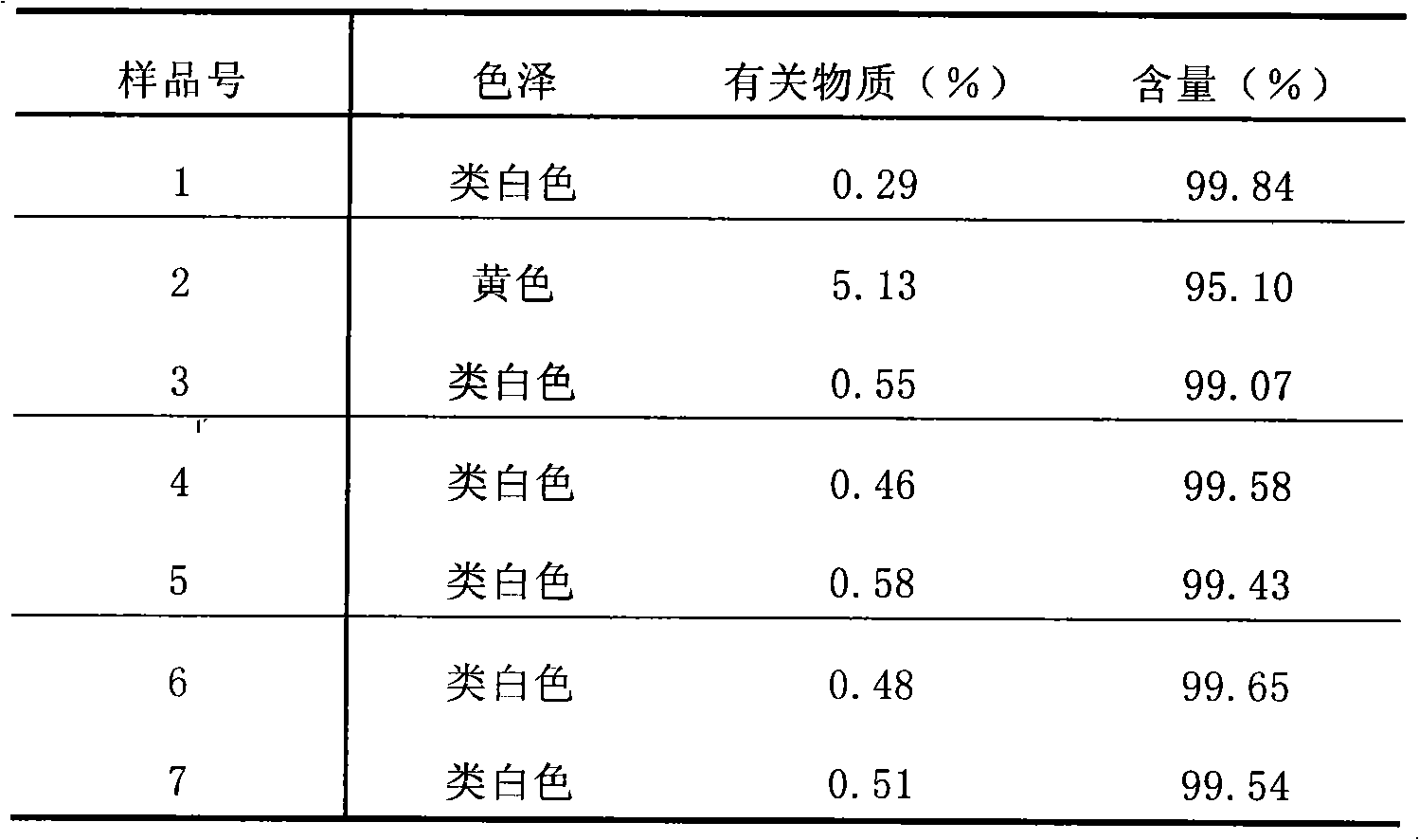
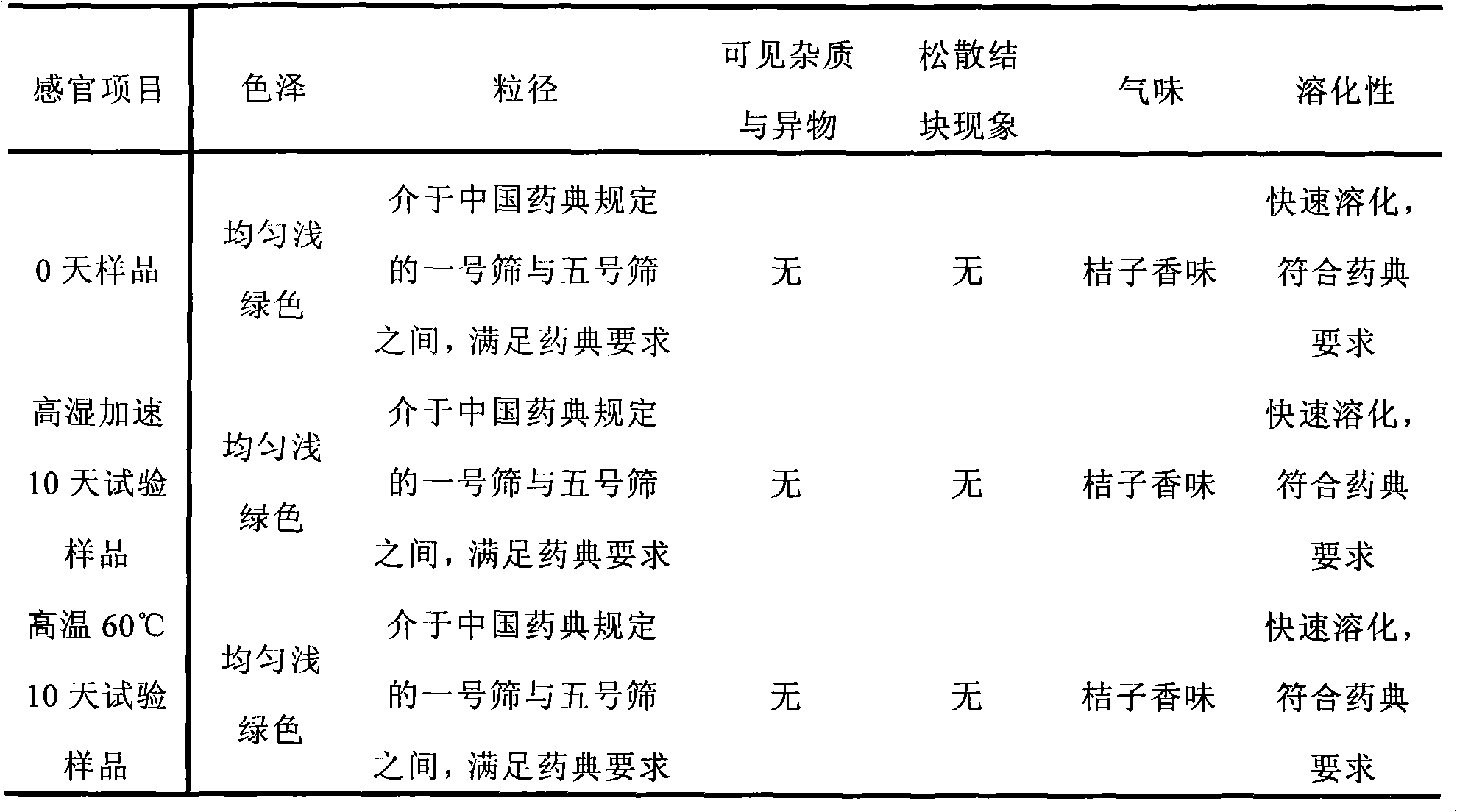
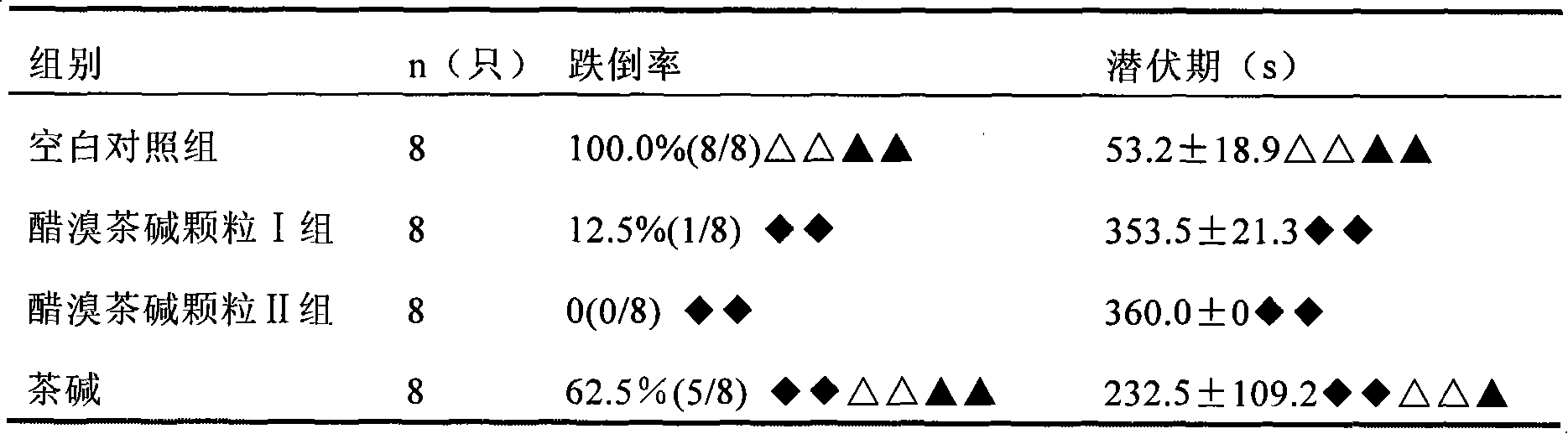
Examples
Embodiment 1
[0038] Embodiment 1: (calculated in 1000 doses)
[0039] Acebrophylline 100g
[0040] Lactose 1710g
[0041] Stevia 150g
[0042] Orange Spice 40g
[0043] Hypromellose solution appropriate amount
[0044] The preparation method is as follows: pulverize and sieve the bromophylline, sieve lactose, stevioside, orange spice, etc., and prepare 1.5% hydroxypropyl methylcellulose and 50% ethanol solution as a wetting adhesive for standby use. Feed according to the above prescription and mix evenly, add wetting adhesive to wet, extrude through a 14-mesh sieve to prepare granules, dry at 50°C, and sieve to select dry granules with a particle size of 180 μm to 2000 μm. After testing, aluminum-plastic Encapsulate and make a sachet, that is, ready.
Embodiment 2
[0045] Embodiment 2: (calculated in 1000 doses)
[0046] Acebrophylline 150g
[0047] Lactose 2000g
[0048] Stevia 150g
[0049] Orange Spice 40g
[0050] Chlorophyll 10mg
[0051] Hypromellose solution appropriate amount
[0052] The preparation method is as follows: pulverize and sieve the bromophylline, sieve lactose, stevia, orange flavor, chlorophyll, etc., and prepare 1.5% hydroxypropyl methylcellulose and 50% ethanol solution as a wetting adhesive for standby use. Feed according to the above prescription and mix evenly, add wetting adhesive to wet, extrude through a 14-mesh sieve to prepare granules, dry at 50°C, and sieve to select dry granules with a particle size of 180 μm to 2000 μm. After testing, aluminum-plastic Encapsulate and make a sachet, that is, ready.
Embodiment 3
[0053] Embodiment 3: (calculated in 1000 doses)
[0054] Bromophylline 50g
[0055] Lactose 1370g
[0056] Stevia 100g
[0057] Banana Flavor 15ml
[0058] Chlorophyll 10mg
[0059] Hypromellose solution appropriate amount
[0060] The preparation method is as follows: pulverize and sieve the bromophylline, sieve lactose and stevioside, prepare 1.5% hydroxypropyl methylcellulose and 50% ethanol solution and mix it with banana essence as a wetting adhesive for standby use. Feed according to the above prescription and mix evenly, add wetting adhesive to wet, extrude through a 14-mesh sieve to prepare granules, dry at 50°C, and sieve to select dry granules with a particle size of 180 μm to 2000 μm. After testing, aluminum-plastic Encapsulate and make a sachet, that is, ready.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com