Carbapenem bicyclic nucleus preparation and purification method
A carbapenem bicyclic nuclei and purification method technology, applied in the direction of organic chemistry, can solve the problems of excessive heavy metal content, affecting the quality of drugs, and no rhodium removal method, etc., to achieve simple operation, cheap reagents, and environmental friendliness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
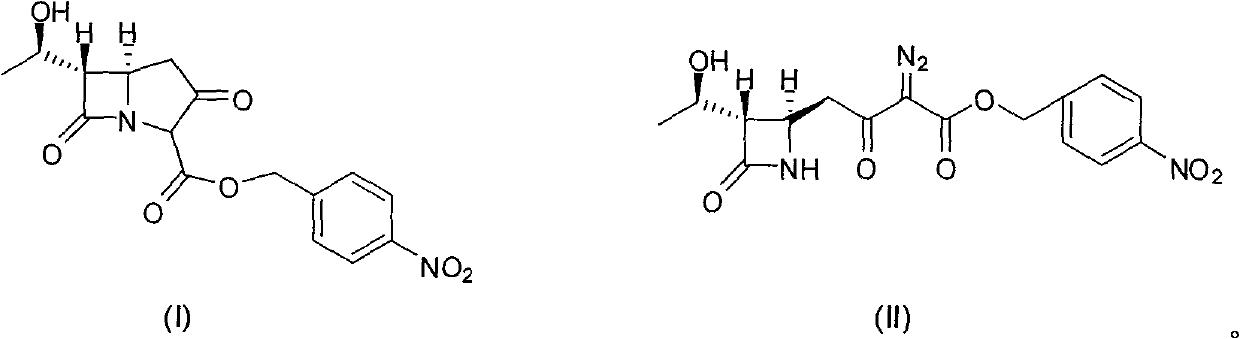
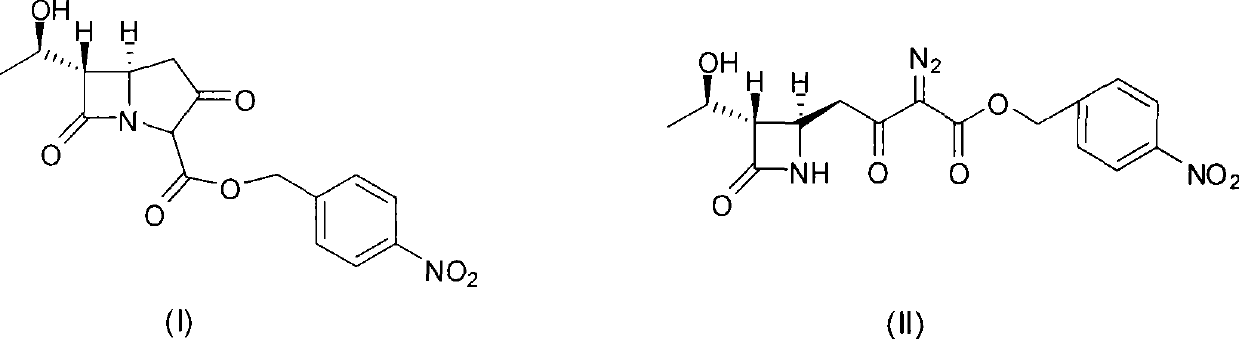
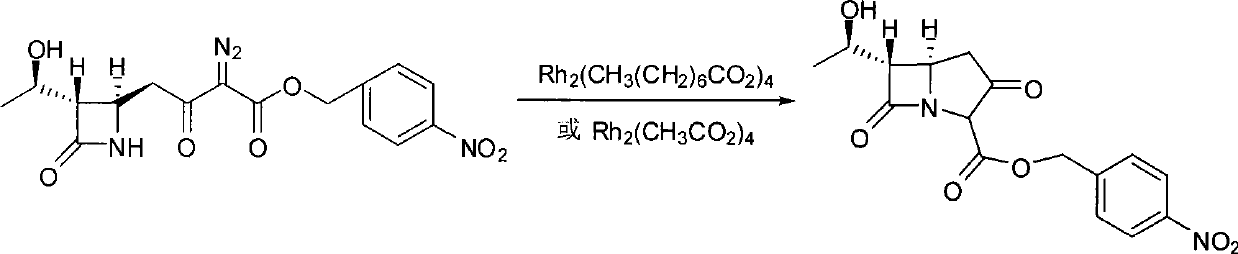
[0029] In a 500ml three-necked flask, add 37.6g (0.1mol) (3S, 4R)-3-[(1R)-hydroxyethyl]-4-[3-(4-nitrobenzyloxy)carbonyl-2-oxo Substitute-3-diazopropyl]azetidinyl-2-one, 300ml ethyl acetate, nitrogen gas for 5min, add 0.39g rhodium octanoate, react at 50°C for 3-4 hours, HPLC detection complete reaction, cooling To room temperature, add 300ml of 5% TMT (pH=7.5~8.0) aqueous solution, stir at room temperature for 30min, static layering, ethyl acetate phase was washed with 200ml of water, dried with anhydrous MgSO4, concentrated ethyl acetate under reduced pressure at less than 50°C Liquid to about 100ml, cooled to room temperature, added 300ml of isopropyl ether, a white solid was precipitated, filtered, and the filter cake was dried under vacuum and reduced pressure at 30-40°C to obtain 33.0g of carbapenem bicyclic nucleus, yield 94.8%, The content determined by HPLC normalization method is 98.7%, and the rhodium content determined by ICP-MS method is 0.6ppm
Embodiment 2
[0031] Except replacing rhodium octanoate with rhodium acetate, the consumption of rhodium acetate is 0.22g, other operating conditions are all identical with embodiment 1, obtain white solid 30.1g, yield 86.5%, HPLC normalization method determines content 99.1%, ICP-MS Determination of rhodium content 0.4ppm.
Embodiment 3
[0033] Except that the consumption of rhodium octanoate is 0.20g, other operating conditions are all the same as in Example 1, and 28.6g of white solid is obtained, and the yield is 82.2%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com