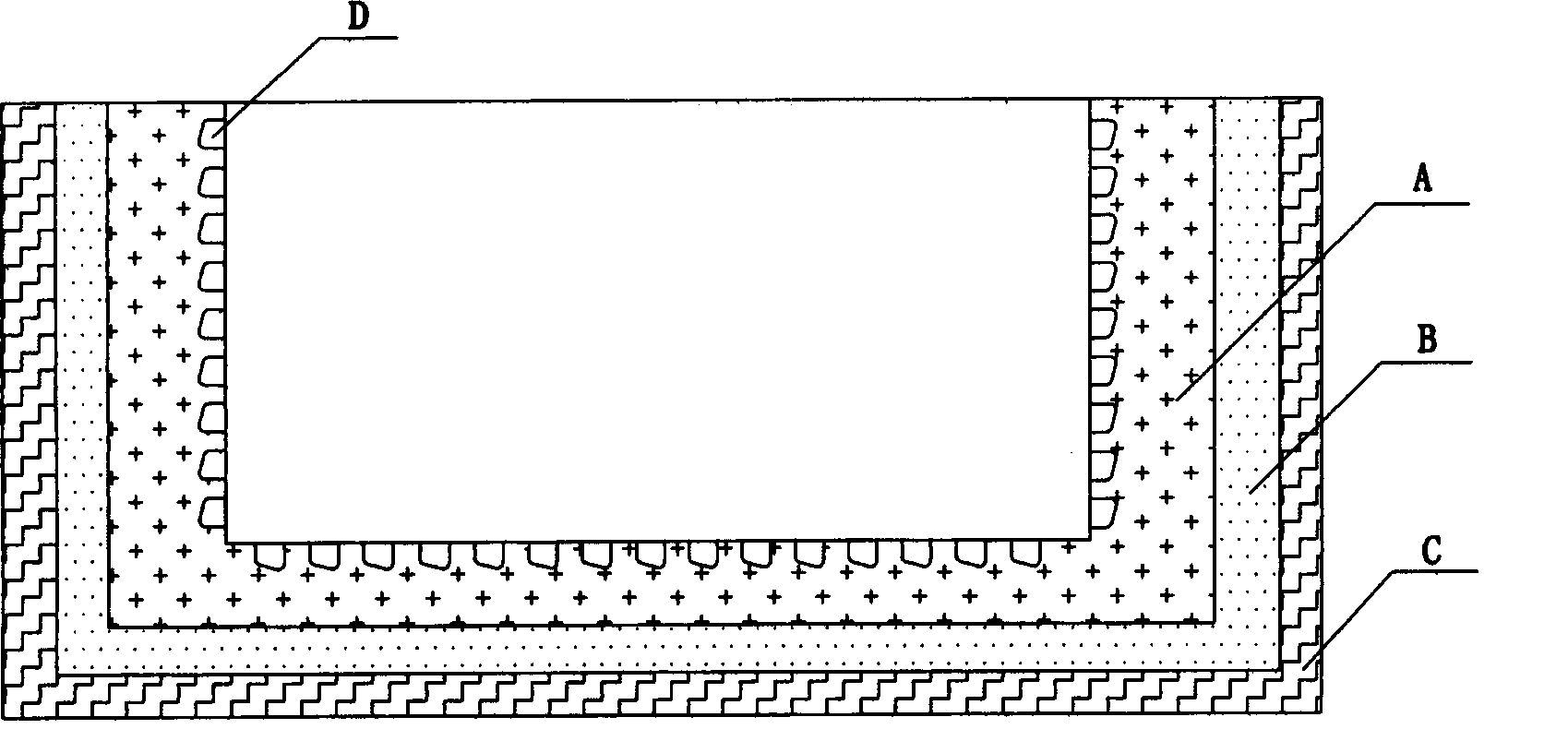
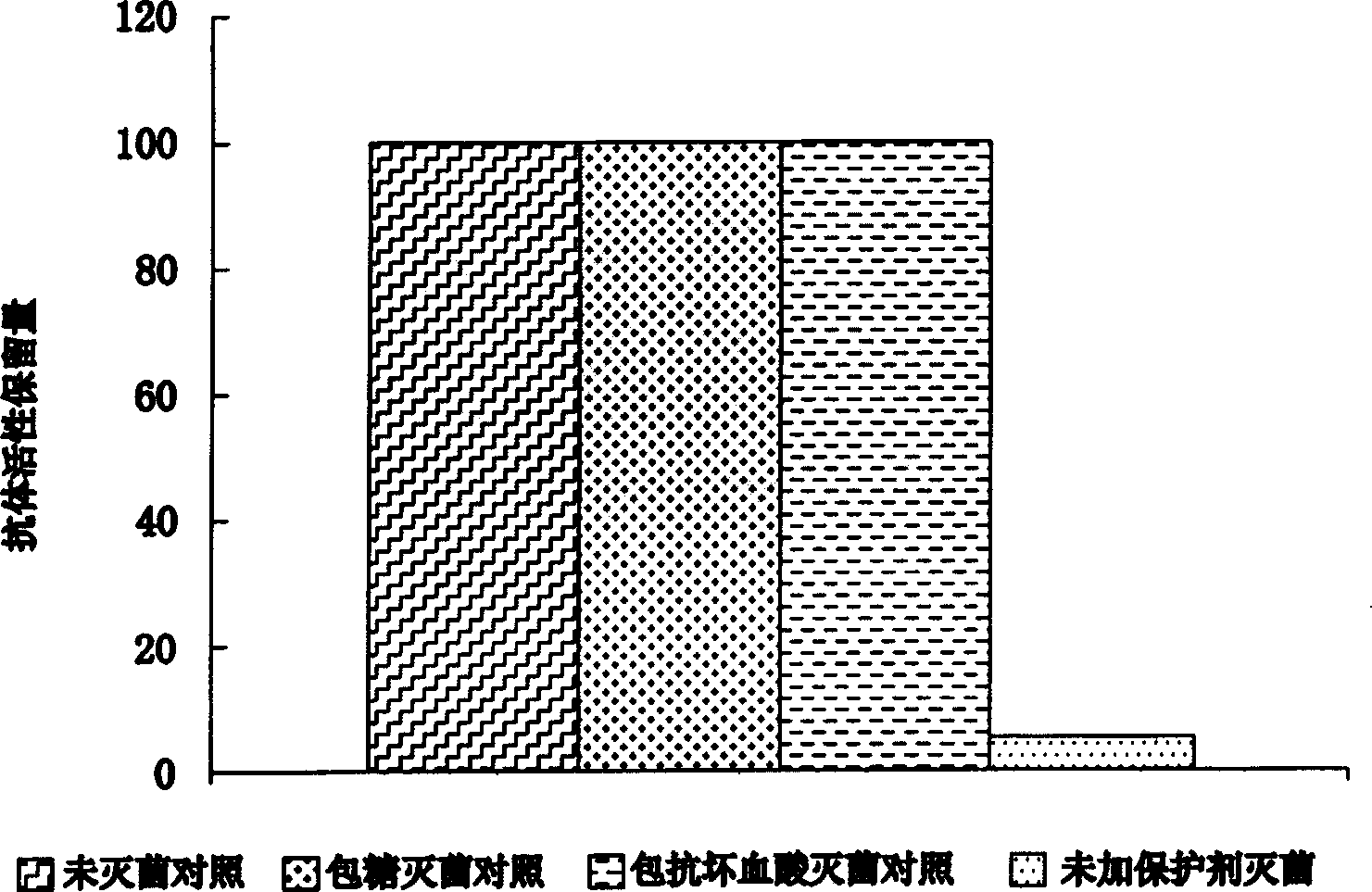
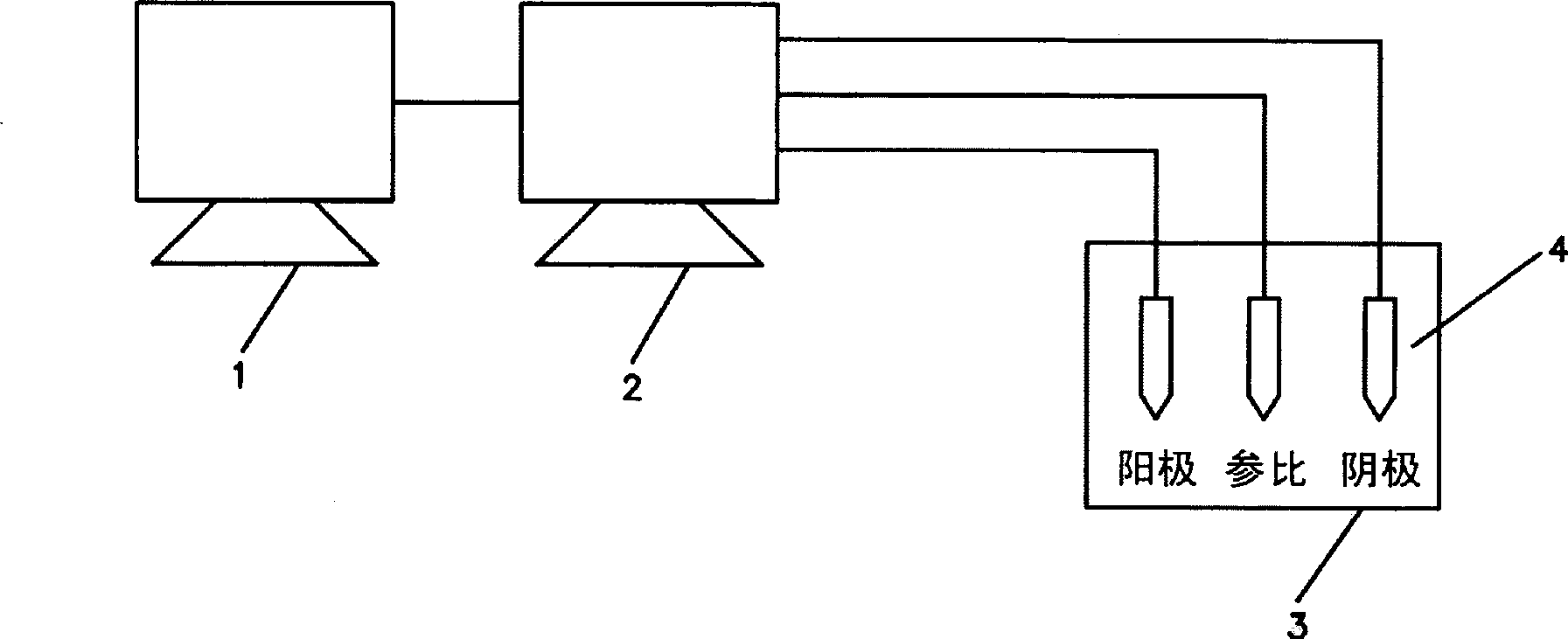
Biocidal treatment method for biological coating medical device
A medical device and sterilization treatment technology, which is applied in the direction of irradiation, etc., can solve the problems of biological active ingredients, biological antibody activity destruction, etc., and achieve excellent sterilization effect, safe use, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of embodiment 1 biocoating medical device
[0038] After polishing, 316 stainless steel alloy or cobalt complex alloy material was used to engrave a vascular stent with a laser, and the monoclonal antibody CD34-coated biological stent was prepared according to the following steps:
[0039] 1. Cleaning of the surface of the bracket body:
[0040] (1) Grinding the surface of the bracket body with an abrasive belt, mechanically removing oxides on the surface, and adjusting the surface roughness;
[0041] (2) Use ultrasonic waves to clean with 75% medical ethanol solution, 99.5% acetone solution, and deionized water in sequence. The ultrasonic frequency is 100KHz, and the cleaning time is 10 minutes;
[0042] (3) Soak the stent body in a 30g / L, 80°C sodium hydroxide solution for 10 minutes to remove grease and scale;
[0043] (4) Place the cleaned stent body in a vacuum dryer, set the temperature at 40° C., and take it out after drying for 45 minutes.
[...
Embodiment 2
[0050] Example 2 Sterilization of biocoated medical devices
[0051] Ascorbate is dissolved in distilled water to obtain an ascorbate solution with a concentration of 20 mg / ml; the ascorbate solution is evenly sprayed on the surface of the biological antibody-coated medical device in Example 1, and the thickness of the sterilization protective coating is about 5 μm ; After pre-freezing the medical device coated with the antibody active protective coating in the refrigerator for 2 hours, put it in a vacuum freeze dryer with a vacuum degree of <10pa and a cold temperature of -55°C for 4 hours, take it out and put it in -20 Store in a ℃ freezer. The packaged medical device is sterilized by Co60-γ-ray irradiation under the conditions of a temperature of 25° C., a dose of 15 KGy, and a humidity of 35%.
Embodiment 3
[0052] The sterilization treatment of embodiment 3 biological coating medical devices
[0053] Dextran 40 was dissolved in distilled water to prepare a dextran 40 solution with a concentration of 20 mg / ml; the dextran 40 solution was evenly sprayed on the surface of the biological antibody-coated medical device in Example 1, and the thickness of the sterilization protective coating was about 5 μm ; After pre-freezing the medical device coated with the antibody active protective coating in the refrigerator for 2 hours, put it in a vacuum freeze dryer with a vacuum degree of <10 Pa and a cooling temperature of -55°C for 4 hours, take it out and put it in -20°C Store in the freezer. The packaged medical device is sterilized by Co60-γ-ray irradiation under the conditions of temperature 25°C, dose 12KGy, and humidity 55%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com