Process for producing luminous rare earth-beta-diketone-polyvinyl pyridine macromolecule composite material
A technology of polyvinylpyridine and composite materials, which is applied in the direction of luminescent materials, chemical instruments and methods, etc., can solve the problem of cancer induction, and achieve the effects of high conversion rate, good reproducibility and good dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
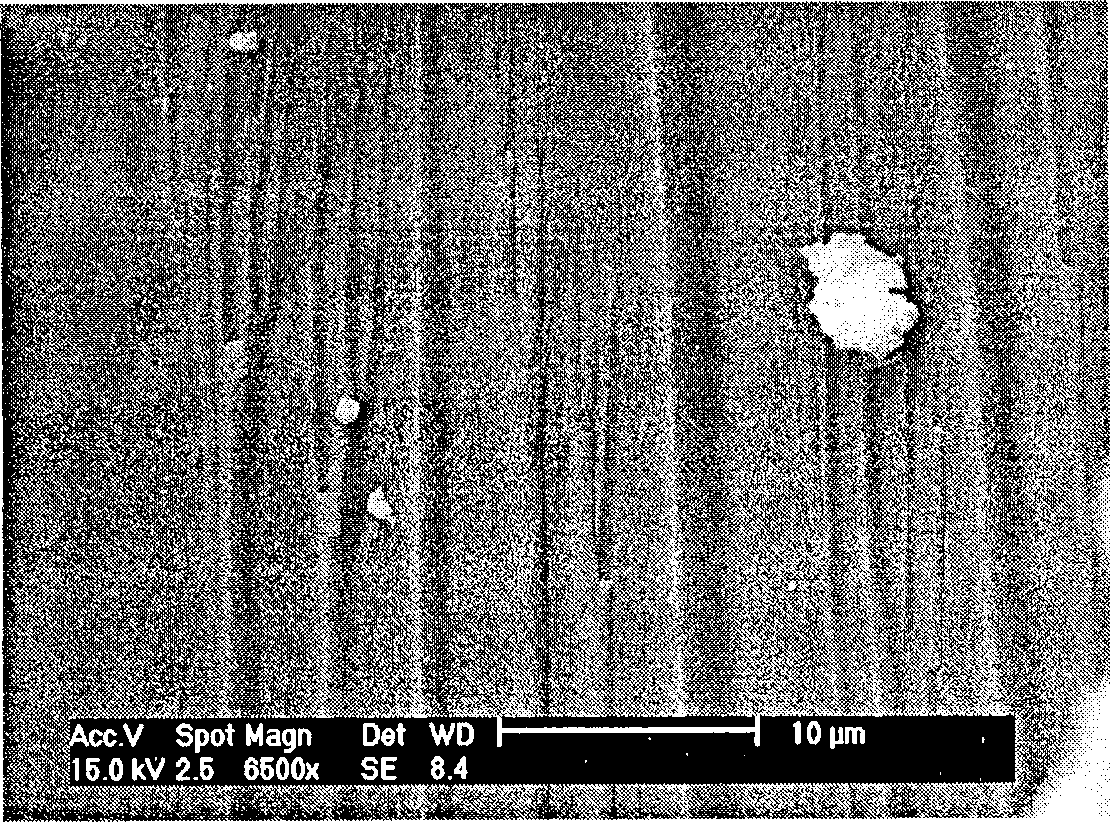
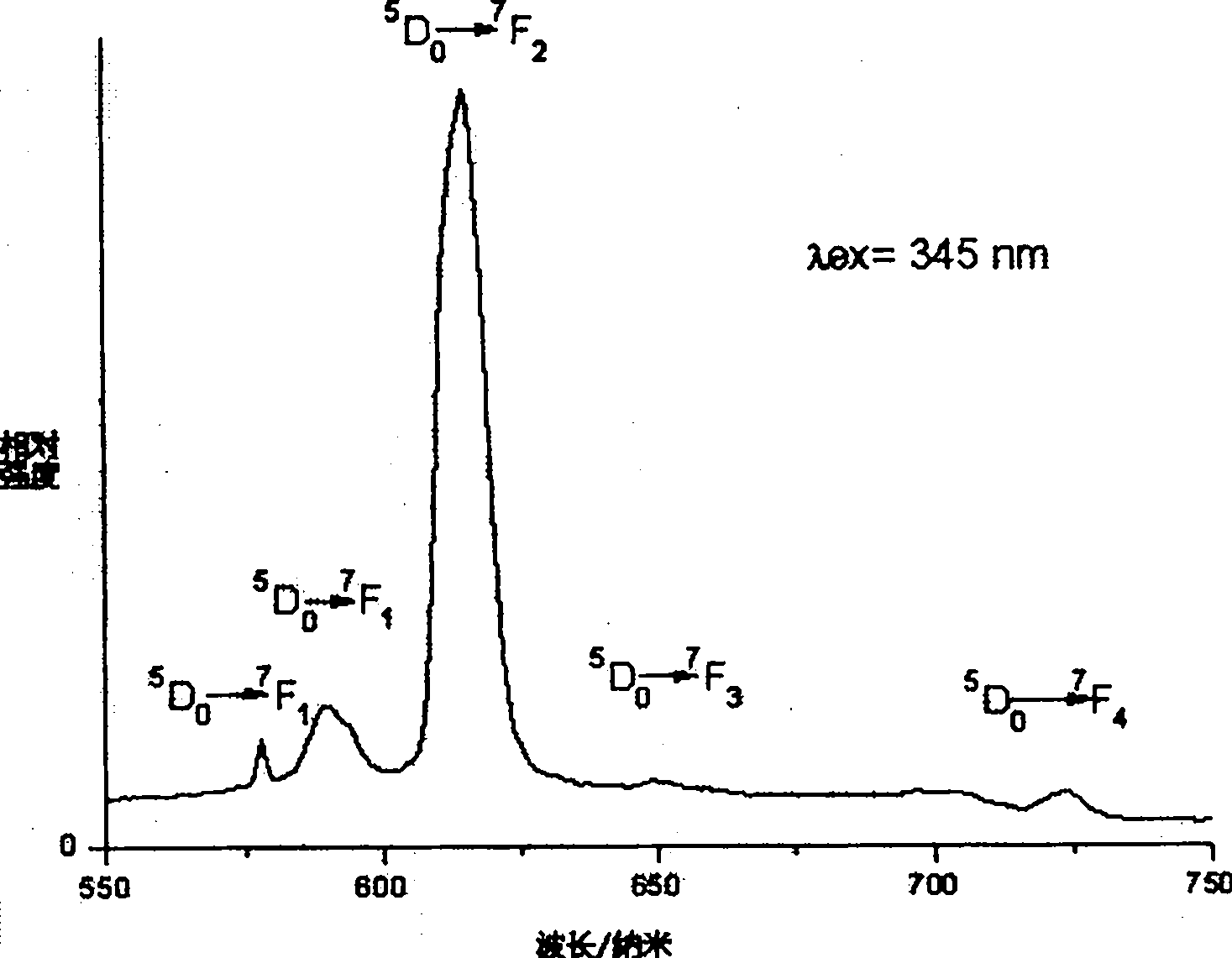
Image
Examples
Embodiment 1
[0033] Dissolve 1.000g of acetylacetone in 15ml of anhydrous acetone and add it to a three-necked flask, then add 5ml of anhydrous acetone solution dissolved with 0.480g of sodium hydride dropwise. The entire solution is controlled at 65°C and heated under nitrogen protection After refluxing for 2 hours, a solution of 4.950 g of triethoxysilylisocyanate dissolved in 5 ml of anhydrous acetone was added dropwise. After the dropwise addition, the temperature of the whole solution was controlled at 60° C., refluxed for 12 h under the protection of nitrogen, then cooled, and the solvent was distilled off under reduced pressure. The resultant was washed three times with 20 ml of cyclohexane to obtain a pale yellow oily liquid. The obtained oily organic bridge molecule system was dissolved in absolute ethanol and set aside. Dissolve 3ml of vinylpyridine in 15ml of anhydrous acetone and add it into a three-necked flask. The temperature of the entire solution is controlled at 60°C, an...
Embodiment 2
[0035] Dissolve 2.243g of dibenzoylmethane in 15ml of anhydrous ether and add it to a three-necked flask, then add 5ml of anhydrous ether solution containing 0.480g of sodium hydride dropwise. After heating to reflux for 2 hours under protection, a solution of 4.950 g of triethoxysilylisocyanate in 5 ml of anhydrous diethyl ether was added dropwise. After the dropwise addition, the temperature of the whole solution was controlled at 65° C., refluxed for 13 h under the protection of nitrogen, then cooled, and the solvent was distilled off under reduced pressure. The resultant was washed three times with 20 ml of cyclohexane to obtain a pale yellow oily liquid. The obtained oily organic bridge molecule system was dissolved in absolute ethanol and set aside. Dissolve 3ml of vinylpyridine in 15ml of anhydrous ether and add it into a three-necked flask. The temperature of the entire solution is controlled at 65°C, and it is refluxed for 7 hours under the protection of nitrogen, th...
Embodiment 3
[0037] Dissolve 2.222g of thienoyltrifluoroacetone in 15ml of anhydrous pyridine and add it to a three-necked flask, then add 5ml of anhydrous pyridine solution with 0.480g of sodium hydride dissolved in it dropwise, and control the entire solution at 75°C. After heating to reflux for 2 hours under the protection of nitrogen, a solution of 4.950 g of triethoxysilylisocyanate in 5 ml of anhydrous pyridine was added dropwise. After the dropwise addition, the temperature of the whole solution was controlled at 70° C., refluxed for 14 h under the protection of nitrogen, then cooled, and the solvent was distilled off under reduced pressure. The resultant was washed three times with 20 ml of cyclohexane to obtain a pale yellow oily liquid. The obtained oily organic bridge molecule system was dissolved in absolute ethanol and set aside. Dissolve 3ml of vinylpyridine in 15ml of anhydrous pyridine and add it into a three-necked flask. The temperature of the entire solution is controll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com