Vaccine for in ovo inoculation
A technology for vaccines and poultry, applied to veterinary vaccines, vaccines, medical preparations containing active ingredients, etc., can solve problems such as taking a very long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] "Example 1: Production of ND vaccine for in ovo vaccination with added virus adsorbent"
[0047] Newcastle disease (ND) vaccines for inoculation in ovo were prepared by adding potassium alum, aluminum hydroxide gel, hydroxyapatite, and silica gel as virus adsorbents. Potassium alum and aluminum hydroxide gels were prepared using physiological saline according to conventional methods, so that the aluminum content was 2 mg / ml, and hydroxyapatite was prepared using physiological saline to be 200 mg / ml. Each 10ml of potassium alum, aluminum hydroxide gel and hydroxyapatite solution was prepared with 10ml of physiological saline to make 10 5.3 EID 50 / ml of the ND virus attenuated strain D26 strain virus solution was mixed, and stirred overnight with a stirrer at 4°C. In addition, 1 g of powdered silica gel and 1 ml of saline were prepared as 10 6.3 EID 50 / ml of ND virus attenuated strain D26 strain virus solution was mixed, added physiological saline to a total of 20ml...
Embodiment 2
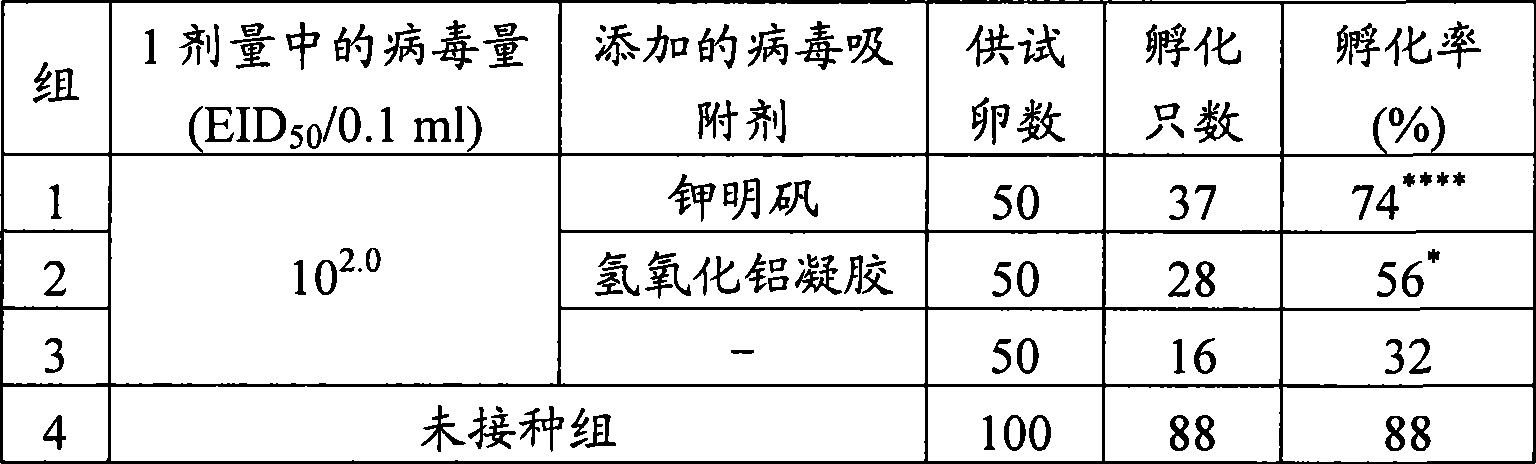
[0051] "Example 2: Safety of ND Vaccine for In-ovo Inoculation with Added Virus Adsorbent in SPF Eggs"
[0052] Using the ND vaccine for in ovo inoculation with the addition of potassium alum and aluminum hydroxide gel prepared according to the method described in Example 1, according to the method reported by Sharma et al. In ovo inoculation of SPF 18-day-old developing chicken eggs (groups 1 and 2). A test group in which no virus adsorbent was added but only virus was set as a control (group 3). As a result, as shown in Table 2, there was a significant difference in hatchability between the ND vaccine group for in-ovo inoculation to which the virus adsorbent was added and the non-addition group (using Fisher's direct probability calculation method to test the significant difference from the non-addition group). ). In addition, there was also a significant difference in the survival rate at the second week after hatching between the ND vaccine group for in ovo inoculation t...
Embodiment 3
[0059] "Example 3: Effectiveness of ND vaccine for in ovo vaccination with added virus adsorbent in SPF eggs"
[0060] In order to evaluate the effectiveness of the test group tested in Example 2, serum was collected at the third week after hatching, and red blood cells were administered using ND virus hemagglutinin (Institute of Chemistry and Serotherapy, Inc.) as described in the attached instruction manual. Agglutination inhibition test (HI test). It is known that generally the HI antibody titer of more than 5 times can defend against the attack of the ND virus virulent strain Sato strain, so the HI antibody titer of the ND vaccine group in ovo inoculation that has added the virus adsorbent is sufficiently higher than the defense level (Table 4).
[0061] [Table 4]
[0062]
[0063] 1) : Antibody positive rate (%) is shown in brackets. It is positive when it is more than 5 times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com