Production of a soluble native form of recombinant protein by the signal sequence and secretional enhancer
A signal sequence and heterologous protein technology, applied in the field of recombinant proteins, can solve the problems of not developing a direct analysis method of signal sequence, impossible and difficult to produce natural form recombinants, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0228] Example 1: Cloning Adhesin Gene DNA Polymeric Cassette
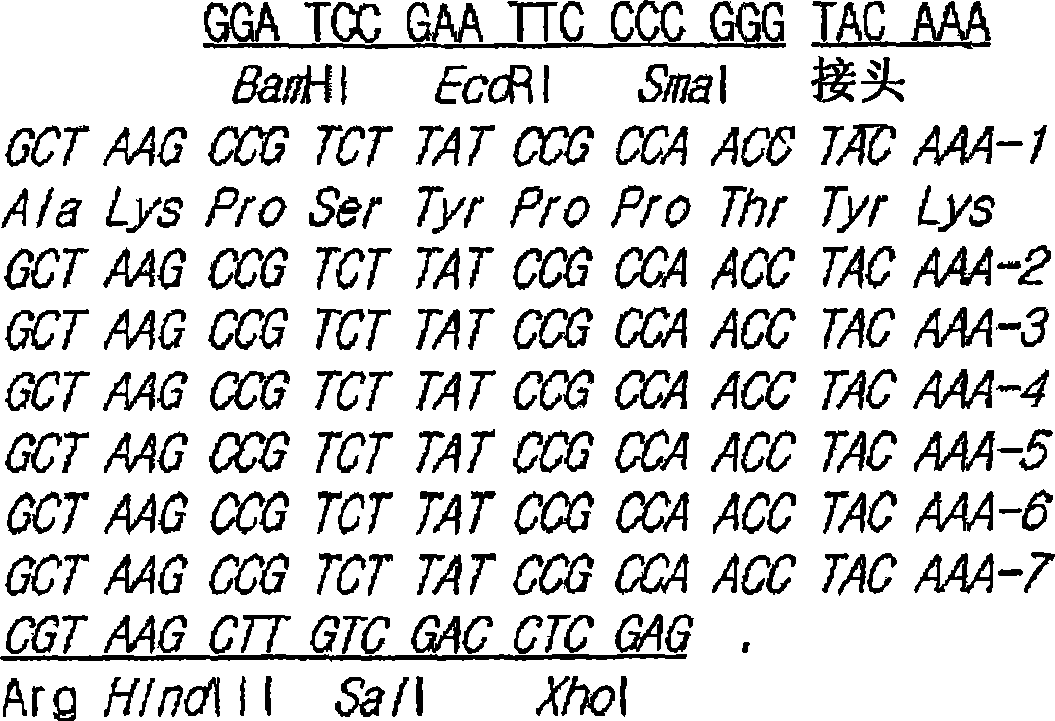
[0229] Based on the basic unit of the Mefp1 amino acid sequence represented by SEQ.ID.NO:1 (Ala Lys Pro Ser Tyr Pro Pro Thr Tyr Lys), by using the basic unit represented by SEQ.ID.NO:2 (5'-TACAAA GCT AAG CCG TCT TAT The forward primer shown in CCG CCA ACC-3') and the reverse primer shown in SEQ.ID.NO:3 (5'-TTT GTA GGT TGG CGG ATA AGA CGG CTTAGC-3') were prepared by the inventors synthesized mefp1 DNA. For the DNA (comprising BamHI / EcoRI / SmaI) synthesized by the left adapter (hereinafter referred to as "La"), the DNA shown by SEQ.ID.NO:4 (5'-GAT CCG AAT TCC CCGGG-3') was used Forward primer and reverse primer shown by SEQ.ID.NO:5 (5'-TTT GTA CCC GGG GAATTC G-3'). For the DNA (comprising Arg / HindIII / SalI / XhoI) synthesized by the right adapter (hereinafter referred to as "Ra"), the DNA prepared from SEQ.ID.NO:6 (5'-TAC AAA CGT AAG CTT GTC GAC C-3 ') and the reverse primer shown by SEQ.ID.NO:7 (5'-TCG AGG TCG ACA...
Embodiment 2
[0250] Example 2: Expression of the adhesion protein mefp1
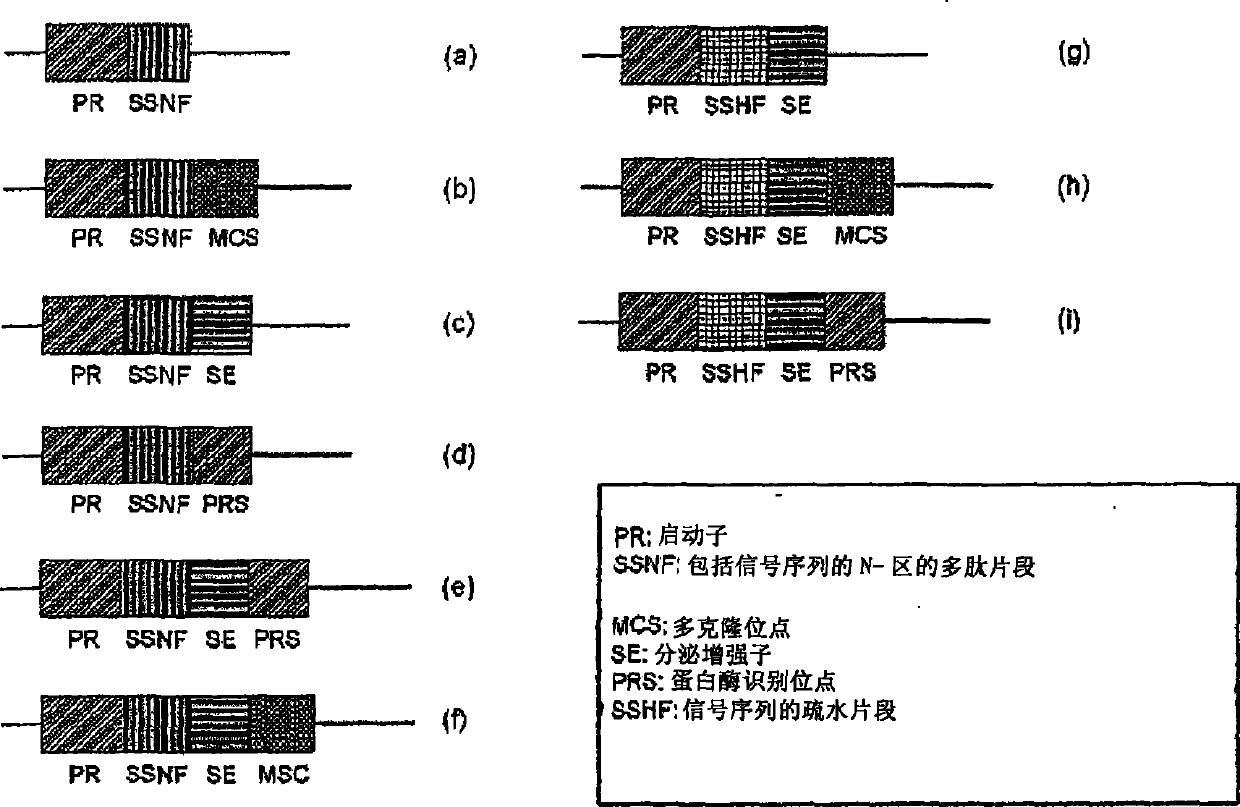
[0251] In a previous study, Mefp1 was expressed as an insoluble inclusion body when Met-Mefp1 was used as a leader sequence (Kitamura et al., J Polym. Sci. Ser. A 37:729-736, 1999). The inventors introduced the signal sequence OmpASP (OmpA signal peptide) to induce the expression of the protein in soluble form, for its use figure 2 The mefp1 sequence was used as a template for PCR to construct clones carrying ompASP and mefp1 cassettes of different sizes (Table 1).
[0252] In the presence of 50 μg / ml of ampicillin at 30°C, the Escherichia coli BL21 (DE3) transformant produced by using the expression vector containing the signal sequence shown in Table 1 was cultured in LB medium (20 g of tryptone, yeast extract Cultivate in 5.0g, NaCl 0.5g, KCl 1.86mg / l) for 16 hours. The culture solution was diluted 200-fold with LB medium. Incubate the diluted culture solution to OD 600 0.3, and then add IPTG to a final con...
Embodiment 3
[0254] Example 3: Production of Adhesin Mefp1 in Native Form
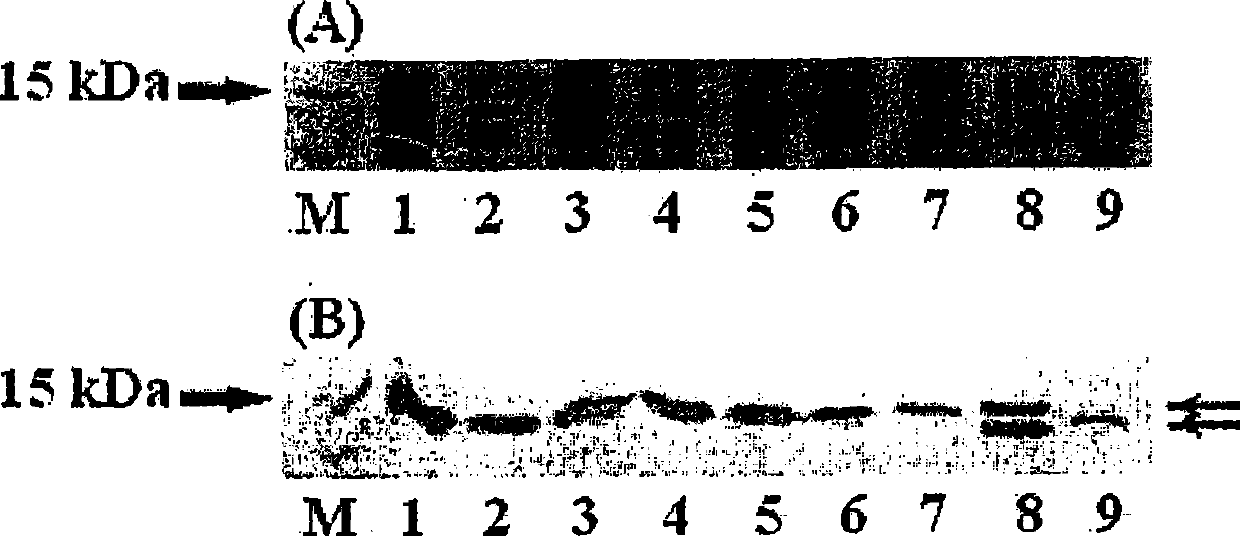
[0255]To produce Mefp1 with its native N-terminus, based on the results of soluble expression by shortened OmpASP (Table 1), the inventors used pBluescriptIISK(+)-La-7×mefp1-Ra( figure 2 ) as a template and synthesized OmpASP encoding a Factor Xa cleavage site for cleaving the C-terminal end 1-8 -Xa-Mefp1 oligonucleotide was used as forward primer for PCR to construct pET-22b(+)(ompASP 1-8 -Xa-7×mefp1 * )( * : Ra-6×His, Ra derived from right adapter; 6×His derived from His tag) clone. Expression of the constructed vectors was checked by transformation and Western blotting as described in Example 2.
[0256] As a result, this clone produced the soluble protein OmpASP 1-8 -Xa-7×Mefp1 * . Furthermore, 7×Mefp1 with native amino acid termini * Protein removal by OmpASP with Factor Xa protease 1-8 -Xa sequence obtained ( Figure 4 ).
[0257] In order to modify the signal sequence region of the above-mention...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com